Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research
- PMID: 23394164
- PMCID: PMC3624074
- DOI: 10.1021/cr3004295
Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research
Figures
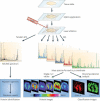
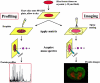

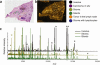
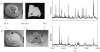
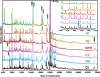
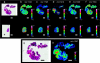



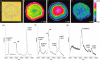

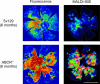
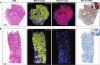

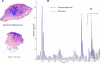
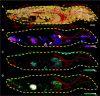
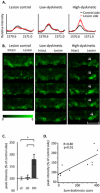

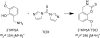
References
-
- Goodwin RJA, Iverson SL, Andren PE. Rapid Commun. Mass Spectrom. 2012;26:494. - PubMed
-
- Kreye F, Hamm G, Karrout Y, Legouffe R, Bonnel D, Siepmann F, Siepmann J. J Control Release. 2012 - PubMed
-
- Schoenheimer R, Rittenberg D. Science. 1935;82:156. - PubMed
-
- Schoenheimer R. The Dynamic State of Body Constituents. Harvard University Press; Cambridge, MA: 1942.
-
- Schoenheimer R, Rittenberg D. Physiol. Rev. 1940;20:218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

