Piroxicam-β-cyclodextrin: a GI safer piroxicam
- PMID: 23394552
- PMCID: PMC3664509
- DOI: 10.2174/09298673113209990115
Piroxicam-β-cyclodextrin: a GI safer piroxicam
Abstract
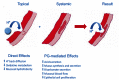
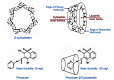
Although NSAIDs are very effective drugs, their use is associated with a broad spectrum of adverse reactions in the liver, kidney, cardiovascular (CV) system, skin and gut. Gastrointestinal (GI) side effects are the most common and constitute a wide clinical spectrum ranging from dyspepsia, heartburn and abdominal discomfort to more serious events such as peptic ulcer with life-threatening complications of bleeding and perforation. The appreciation that CV risk is also increased further complicates the choices of physicians prescribing anti-inflammatory therapy. Despite prevention strategies should be implemented in patients at risk, gastroprotection is often underused and adherence to treatment is generally poor. A more appealing approach would be therefore to develop drugs that are devoid of or have reduced GI toxicity. Gastro- duodenal mucosa possesses many defensive mechanisms and NSAIDs have a deleterious effect on most of them. This results in a mucosa less able to cope with even a reduced acid load. NSAIDs cause gastro-duodenal damage, by two main mechanisms: a physiochemical disruption of the gastric mucosal barrier and systemic inhibition of gastric mucosal protection, through inhibition of cyclooxygenase (COX, PG endoperoxide G/H synthase) activity of the GI mucosa. However, against a background of COX inhibition by anti-inflammatory doses of NSAIDs, their physicochemical properties, in particular their acidity, underlie the topical effect leading to short-term damage. It has been shown that esterification of acidic NSAIDs suppresses their gastrotoxicity without adversely affecting anti-inflammatory activity. Another way to develop NSAIDs with better GI tolerability is to complex these molecules with cyclodextrins (CDs), giving rise to so-called "inclusion complexes" that can have physical, chemical and biological properties very different from either those of the drug or the cyclodextrin. Complexation of NSAIDs with β-cyclodextrin potentially leads to a more rapid onset of action after oral administration and improved GI tolerability because of minimization of the drug gastric effects. One such drug, piroxicam-β-cyclodextrin (PBC), has been used in Europe for 25 years. Preclinical and clinical pharmacology of PBC do show that the β-cyclodextrin inclusion complex of piroxicam is better tolerated from the upper GI tract than free piroxicam, while retaining all the analgesic and anti-inflammatory properties of the parent compound. In addition, the drug is endowed with a quick absorption rate, which translates into a faster onset of analgesic activity, an effect confirmed in several clinical studies. An analysis of the available trials show that PBC has a GI safety profile, which is better than that displayed by uncomplexed piroxicam. Being an inclusion complex of piroxicam, whose CV safety has been pointed out by several observational studies, PBC should be viewed as a CV safe anti-inflmmatory compound and a GI safer alternative to piroxicam. As a consequence, it should be considered as a useful addition to our therapeutic armamentarium.
Figures



References
-
- Aronson JK. Meyler's Side Effects of Analgesics and Anti-Inflammatory Drugs. Amsterdam: Elsevier; 2009. pp. 1–500.
-
- Lanas A, Hunt R. Prevention of anti-inflammatory drug-induced gastrointestinal damage benefits and risks of therapeutic strategies. Ann. Med . 2006;38: 415–428. - PubMed
-
- Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol. Clin. North Am . 2010;39:433–464. - PubMed
-
- Chan FK, Abraham NS, Scheiman JM, Laine L. Management of patients on nonsteroidal anti-inflammatory drugs: a clinical practice recommendation from the First International Working Party on Gastrointestinal and Cardiovascular Effects of Nonsteroidal Anti-inflammatory Drugs and Anti-platelet Agents. Am. J. Gastroenterol . 2008;103:2908–2918. - PubMed
-
- Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol . 2009;104:728–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
