Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer
- PMID: 23395006
- PMCID: PMC3650477
- DOI: 10.1016/j.pec.2012.12.014
Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer
Abstract
Objective: To assess the impact of Guide to Decide (GtD), a web-based, personally-tailored decision aid designed to inform women's decisions about prophylactic tamoxifen and raloxifene use.
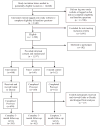
Methods: Postmenopausal women, age 46-74, with BCRAT 5-year risk ≥ 1.66% and no prior history of breast cancer were randomized to one of three study arms:intervention (n=690), Time 1 control (n=160), or 3-month control (n=162). Intervention participants viewed GtD prior to completing a post-test and 3 month follow-up assessment. Controls did not. We assessed the impact of GtD on women's decisional conflict levels and treatment decision behavior at post-test and at 3 months, respectively.
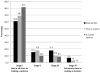
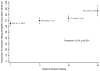
Results: Intervention participants had significantly lower decisional conflict levels at post-test (p<0.001) and significantly higher odds of making a decision about whether or not to take prophylactic tamoxifen or raloxifene at 3-month follow-up (p<0.001) compared to control participants.
Conclusion: GtD lowered decisional conflict and helped women at high risk of breast cancer decide whether to take prophylactic tamoxifen or raloxifene to reduce their cancer risk.
Practice implications: Web-based, tailored decision aids should be used more routinely to facilitate informed medical decisions, reduce patients' decisional conflict, and empower patients to choose the treatment strategy that best reflects their own values.
Trial registration: ClinicalTrials.gov NCT00967824.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
None declared.
Figures
References
-
- Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–8. - PubMed
-
- Domchek SM, Eisen A, Calzone K, Stopfer J, Blackwood A, Weber BL. Application of breast cancer risk prediction models in clinical practice. J Clin Oncol. 2003;21:593–601. - PubMed
-
- Bevers TB, Armstrong DK, Arun B, Carlson RW, Cowan KH, Daly MB, et al. Breast cancer risk reduction. J Natl Compr Canc Netw. 2010;8:1112–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

