MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16
- PMID: 23395168
- PMCID: PMC3641657
- DOI: 10.1016/j.cmet.2013.01.004
MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16
Abstract
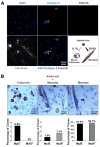
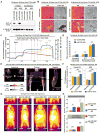
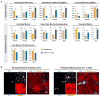
Brown adipose tissue (BAT) is an energy-dispensing thermogenic tissue that plays an important role in balancing energy metabolism. Lineage-tracing experiments indicate that brown adipocytes are derived from myogenic progenitors during embryonic development. However, adult skeletal muscle stem cells (satellite cells) have long been considered uniformly determined toward the myogenic lineage. Here, we report that adult satellite cells give rise to brown adipocytes and that microRNA-133 regulates the choice between myogenic and brown adipose determination by targeting the 3'UTR of Prdm16. Antagonism of microRNA-133 during muscle regeneration increases uncoupled respiration, glucose uptake, and thermogenesis in local treated muscle and augments whole-body energy expenditure, improves glucose tolerance, and impedes the development of diet-induced obesity. Finally, we demonstrate that miR-133 levels are downregulated in mice exposed to cold, resulting in de novo generation of satellite cell-derived brown adipocytes. Therefore, microRNA-133 represents an important therapeutic target for the treatment of obesity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. - PubMed
-
- Bowman WC, Nott MW. Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol Rev. 1969;21:27–72. - PubMed
-
- Cannon B, Nedergaard J. Respiratory and thermogenic capacities of cells and mitochondria from brown and white adipose tissue. Methods Mol Biol. 2001;155:295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

