The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption
- PMID: 23395174
- PMCID: PMC3612289
- DOI: 10.1016/j.cmet.2013.01.007
The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption
Abstract
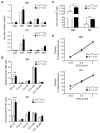
Red blood cell production is a finely tuned process that requires coordinated oxygen- and iron-dependent regulation of cell differentiation and iron metabolism. Here, we show that translational regulation of hypoxia-inducible factor 2α (HIF-2α) synthesis by iron regulatory protein 1 (IRP1) is critical for controlling erythrocyte number. IRP1-null (Irp1(-/-)) mice display a marked transient polycythemia. HIF-2α messenger RNA (mRNA) is derepressed in kidneys of Irp1(-/-) mice but not in kidneys of Irp2(-/-) mice, leading to increased renal erythropoietin (Epo) mRNA and inappropriately elevated serum Epo levels. Expression of the iron transport genes DCytb, Dmt1, and ferroportin, as well as other HIF-2α targets, is enhanced in Irp1(-/-) duodenum. Analysis of mRNA translation state in the liver revealed IRP1-dependent dysregulation of HIF-2α mRNA translation, whereas IRP2 deficiency derepressed translation of all other known 5' iron response element (IRE)-containing mRNAs expressed in the liver. These results uncover separable physiological roles of each IRP and identify IRP1 as a therapeutic target for manipulating HIF-2α action in hematologic, oncologic, and other disorders.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

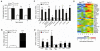

References
-
- Andrews NC, Bridges KR. Disorders of iron metabolism and sideroblastic anemia. In: Nathan DG, Oski SH, editors. Nathan and Oski’s Hematology in Infancy and Childhood. W.B. Saunders; Philadelphia, PA: 1998. pp. 423–461.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM102756/GM/NIGMS NIH HHS/United States
- R01 DK066600/DK/NIDDK NIH HHS/United States
- R01 DK66600/DK/NIDDK NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- R56 DK066600/DK/NIDDK NIH HHS/United States
- R01 DK 080011/DK/NIDDK NIH HHS/United States
- 1UL1RR025011/RR/NCRR NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- R01 DK080011/DK/NIDDK NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- R56 DK080011/DK/NIDDK NIH HHS/United States
- R01CA152108/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

