Understanding metabolic regulation and its influence on cell physiology
- PMID: 23395269
- PMCID: PMC3569837
- DOI: 10.1016/j.molcel.2013.01.018
Understanding metabolic regulation and its influence on cell physiology
Abstract
Metabolism impacts all cellular functions and plays a fundamental role in biology. In the last century, our knowledge of metabolic pathway architecture and the genomic landscape of disease has increased exponentially. Combined with these insights, advances in analytical methods for quantifying metabolites and systems approaches to analyze these data now provide powerful tools to study metabolic regulation. Here we review the diverse mechanisms cells use to adapt metabolism to specific physiological states and discuss how metabolic flux analyses can be applied to identify important regulatory nodes to understand normal and pathological cell physiology.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
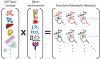
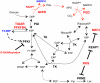
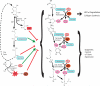
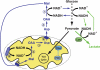
References
-
- Albe KR, Butler MH, Wright BE. Cellular concentrations of enzymes and their substrates. J Theor Biol. 1990;143:163–195. - PubMed
-
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab Eng. 2006;8:324–337. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

