Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults
- PMID: 23395677
- PMCID: PMC3626102
- DOI: 10.1016/j.immuni.2012.10.021
Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults
Abstract
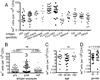
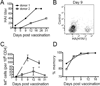
Although T cell memory is generally thought to require direct antigen exposure, we found an abundance of memory-phenotype cells (20%-90%, averaging over 50%) of CD4(+) T cells specific to viral antigens in adults who had never been infected. These cells express the appropriate memory markers and genes, rapidly produce cytokines, and have clonally expanded. In contrast, the same T cell receptor (TCR) specificities in newborns are almost entirely naïve, which might explain the vulnerability of young children to infections. One mechanism for this phenomenon is TCR cross-reactivity to environmental antigens, and in support of this, we found extensive cross-recognition by HIV-1 and influenza-reactive T lymphocytes to other microbial peptides and expansion of one of these after influenza vaccination. Thus, the presence of these memory-phenotype T cells has significant implications for immunity to novel pathogens, child and adult health, and the influence of pathogen-rich versus hygienic environments.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





Comment in
-
T cells: Memories of the future.Nat Rev Immunol. 2013 Mar;13(3):152-3. doi: 10.1038/nri3411. Nat Rev Immunol. 2013. PMID: 23435321 No abstract available.
-
One bug or another: promiscuous T cells form lifelong memory.Immunity. 2013 Feb 21;38(2):207-8. doi: 10.1016/j.immuni.2013.02.002. Immunity. 2013. PMID: 23438820
References
-
- Aaby P, Jensen H, Samb B, Cisse B, Sodemann M, Jakobsen M, Poulsen A, Rodrigues A, Lisse IM, Simondon F, Whittle H. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. Lancet. 2003;361:2183–2188. - PubMed
-
- Alexander J, Bilsel P, del Guercio MF, Stewart S, Marinkovic-Petrovic A, Southwood S, Crimi C, Vang L, Walker L, Ishioka G, et al. Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice. Vaccine. 2010;28:664–672. - PMC - PubMed
-
- Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

