Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau
- PMID: 23395797
- PMCID: PMC3678381
- DOI: 10.1016/j.febslet.2013.01.051
Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau
Erratum in
- FEBS Lett. 2013 Aug 2;587(15):2484
Abstract
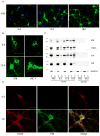
Increasing evidence demonstrates the transmissibility of fibrillar species of tau protein, but this has never been directly tested in neurons, the cell type most affected by formation of tau inclusions in neurodegenerative tauopathies. Here we show that synthetic tau fibrils made from recombinant protein not only time-dependently recruit normal tau into neurofibrillary tangle-like insoluble aggregates in primary hippocampal neurons over-expressing human tau, but also induce neuritic tau pathology in non-transgenic neurons. This study provides highly compelling support for the protein-only hypothesis of pathological tau transmission in primary neurons and describes a useful neuronal model for studying the pathogenesis of tauopathies.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature reviews Neuroscience. 2007;8:663–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

