Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine
- PMID: 23395862
- PMCID: PMC3622758
- DOI: 10.1016/j.neuroscience.2013.01.063
Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine
Abstract
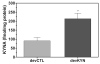
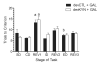
Levels of kynurenic acid (KYNA), an endogenous α7 nicotinic acetylcholine receptor (α7nAChR) antagonist, are elevated in the brain of patients with schizophrenia (SZ) and might contribute to the pathophysiology and cognitive deficits seen in the disorder. As developmental vulnerabilities contribute to the etiology of SZ, we determined, in rats, the effects of perinatal increases in KYNA on brain chemistry and cognitive flexibility. KYNA's bioprecursor l-kynurenine (100mg/day) was fed to dams from gestational day 15 to postnatal day 21 (PD21). Offspring were then given regular chow until adulthood. Control rats received unadulterated mash. Brain tissue levels of KYNA were measured at PD2 and PD21, and extracellular levels of KYNA and glutamate were determined by microdialysis in the prefrontal cortex in adulthood (PD56-80). In other adult rats, the effects of perinatal l-kynurenine administration on cognitive flexibility were assessed using an attentional set-shifting task. l-Kynurenine treatment raised forebrain KYNA levels ∼3-fold at PD2 and ∼2.5-fold at PD21. At PD56-80, extracellular prefrontal KYNA levels were moderately but significantly elevated (+12%), whereas extracellular glutamate levels were not different from controls. Set-shifting was selectively impaired by perinatal exposure to l-kynurenine, as treated rats acquired the discrimination and intra-dimensional shift at the same rate as controls, yet exhibited marked deficits in the initial reversal and extra-dimensional shift. Acute administration of the α7nAChR-positive modulator galantamine (3.0mg/kg, i.p.) restored performance to control levels. These results validate early developmental exposure to l-kynurenine as a novel, naturalistic animal model for studying cognitive deficits in SZ.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




References
-
- Alexander KS, Bortz DM, Wu HQ, Brooks JM, Schwarcz R, Bruno JP. Perinatal elevations of kynurenic acid dysregulate prefrontal glutamate release and produce set-shifting deficits in adults: a new model of schizophrenia. Soc. Neurosci. Abstr. 2011;36:163.23.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

