Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype
- PMID: 23395910
- PMCID: PMC3633821
- DOI: 10.1096/fj.12-223404
Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype
Abstract
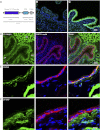
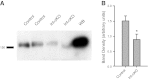
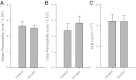
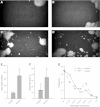
Bladder urothelium senses and communicates information about bladder fullness. However, the mechanoreceptors that respond to tissue stretch are poorly defined. Integrins are mechanotransducers in other tissues. Therefore, we eliminated β1-integrin selectively in urothelium of mice using Cre-LoxP targeted gene deletion. β1-Integrin localized to basal/intermediate urothelial cells by confocal microscopy. β1-Integrin conditional-knockout (β1-cKO) mice lacking urothelial β1-integrin exhibited down-regulation and mislocalization of α3- and α5-integrins by immunohistochemistry but, surprisingly, had normal morphology, permeability, and transepithelial resistance when compared with Cre-negative littermate controls. β1-cKO mice were incontinent, as judged by random urine leakage on filter paper (4-fold higher spotting, P<0.01; 2.5-fold higher urine area percentage, P<0.05). Urodynamic function assessed by cystometry revealed bladder overfilling with 80% longer intercontractile intervals (P<0.05) and detrusor hyperactivity (3-fold more prevoid contractions, P<0.05), but smooth muscle contractility remained intact. ATP secretion into the lumen was elevated (49 vs. 22 nM, P<0.05), indicating abnormal filling-induced purinergic signaling, and short-circuit currents (measured in Ussing chambers) revealed 2-fold higher stretch-activated ion channel conductances in response to hydrostatic pressure of 1 cmH2O (P<0.05). We conclude that loss of integrin signaling from urothelium results in incontinence and overactive bladder due to abnormal mechanotransduction; more broadly, our findings indicate that urothelium itself directly modulates voiding.
Figures



Comment in
-
Incontinence: urothelial β1-integrin knockout suggests mechanosensory mechanism for overactive bladder.Nat Rev Urol. 2013 Apr;10(4):185. doi: 10.1038/nrurol.2013.36. Epub 2013 Feb 26. Nat Rev Urol. 2013. PMID: 23443015 No abstract available.
-
Re: Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype.J Urol. 2013 Dec;190(6):2305. doi: 10.1016/j.juro.2013.08.055. Epub 2013 Aug 30. J Urol. 2013. PMID: 24209569 No abstract available.
-
Re: Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype.J Urol. 2014 Mar;191(3):869-70. doi: 10.1016/j.juro.2013.11.093. Epub 2013 Dec 3. J Urol. 2014. PMID: 24522081 No abstract available.
Similar articles
-
Re: Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype.J Urol. 2014 Mar;191(3):869-70. doi: 10.1016/j.juro.2013.11.093. Epub 2013 Dec 3. J Urol. 2014. PMID: 24522081 No abstract available.
-
Re: Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype.J Urol. 2013 Dec;190(6):2305. doi: 10.1016/j.juro.2013.08.055. Epub 2013 Aug 30. J Urol. 2013. PMID: 24209569 No abstract available.
-
Functional roles for PIEZO1 and PIEZO2 in urothelial mechanotransduction and lower urinary tract interoception.JCI Insight. 2021 Oct 8;6(19):e152984. doi: 10.1172/jci.insight.152984. JCI Insight. 2021. PMID: 34464353 Free PMC article.
-
A Comprehensive Review of Overactive Bladder Pathophysiology: On the Way to Tailored Treatment.Eur Urol. 2019 Jun;75(6):988-1000. doi: 10.1016/j.eururo.2019.02.038. Epub 2019 Mar 26. Eur Urol. 2019. PMID: 30922690 Review.
-
The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder.BJU Int. 2010 Oct;106(8):1114-27. doi: 10.1111/j.1464-410X.2010.09650.x. BJU Int. 2010. PMID: 21156013 Review.
Cited by
-
Interactions of DPP-4 and integrin β1 influences endothelial-to-mesenchymal transition.Kidney Int. 2015 Sep;88(3):479-89. doi: 10.1038/ki.2015.103. Epub 2015 Apr 1. Kidney Int. 2015. PMID: 25830763
-
Exploring Integrins in Smooth Muscle Function: Addressing Challenging Levels of Complexity and Deciphering Organ-specific Codes of Contraction?Function (Oxf). 2022 Oct 15;3(6):zqac053. doi: 10.1093/function/zqac053. eCollection 2022. Function (Oxf). 2022. PMID: 36381383 Free PMC article. No abstract available.
-
Control of urinary drainage and voiding.Clin J Am Soc Nephrol. 2015 Mar 6;10(3):480-92. doi: 10.2215/CJN.04520413. Epub 2014 Apr 17. Clin J Am Soc Nephrol. 2015. PMID: 24742475 Free PMC article. Review.
-
Prostatic inflammation induces urinary frequency in adult mice.PLoS One. 2015 Feb 3;10(2):e0116827. doi: 10.1371/journal.pone.0116827. eCollection 2015. PLoS One. 2015. PMID: 25647072 Free PMC article.
-
Molecular mechanisms of voiding dysfunction in a novel mouse model of acute urinary retention.FASEB J. 2021 Apr;35(4):e21447. doi: 10.1096/fj.202002415R. FASEB J. 2021. PMID: 33742688 Free PMC article.
References
-
- De Groat W. C. (2004) The urothelium in overactive bladder: passive bystander or active participant? Urology 64, 7–11 - PubMed
-
- Apodaca G., Balestreire E., Birder L. A. (2007) The uroepithelial-associated sensory web. Kidney Int. 72, 1057–1064 - PubMed
-
- Wang E. C., Lee J. M., Johnson J. P., Kleyman T. R., Bridges R., Apodaca G. (2003) Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am. J. Physiol. Renal Physiol. 285, F651–F663 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

