RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis
- PMID: 23396135
- PMCID: PMC4245152
- DOI: 10.1038/ng.2541
RNF212 is a dosage-sensitive regulator of crossing-over during mammalian meiosis
Abstract
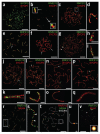
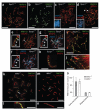
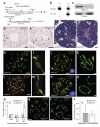
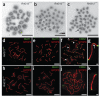
Crossing-over ensures accurate chromosome segregation during meiosis, and every pair of chromosomes obtains at least one crossover, even though the majority of recombination sites yield non-crossovers. A putative regulator of crossing-over is RNF212, which is associated with variation in crossover rates in humans. We show that mouse RNF212 is essential for crossing-over, functioning to couple chromosome synapsis to the formation of crossover-specific recombination complexes. Selective localization of RNF212 to a subset of recombination sites is shown to be a key early step in the crossover designation process. RNF212 acts at these sites to stabilize meiosis-specific recombination factors, including the MutSγ complex (MSH4-MSH5). We infer that selective stabilization of key recombination proteins is a fundamental feature of meiotic crossover control. Haploinsufficiency indicates that RNF212 is a limiting factor for crossover control and raises the possibility that human alleles may alter the amount or stability of RNF212 and be risk factors for aneuploid conditions.
Figures


Similar articles
-
Antagonistic roles of ubiquitin ligase HEI10 and SUMO ligase RNF212 regulate meiotic recombination.Nat Genet. 2014 Feb;46(2):194-9. doi: 10.1038/ng.2858. Epub 2014 Jan 5. Nat Genet. 2014. PMID: 24390283 Free PMC article.
-
Crossover recombination and synapsis are linked by adjacent regions within the N terminus of the Zip1 synaptonemal complex protein.PLoS Genet. 2019 Jun 20;15(6):e1008201. doi: 10.1371/journal.pgen.1008201. eCollection 2019 Jun. PLoS Genet. 2019. PMID: 31220082 Free PMC article.
-
Distinct and interdependent functions of three RING proteins regulate recombination during mammalian meiosis.Proc Natl Acad Sci U S A. 2025 Jan 14;122(2):e2412961121. doi: 10.1073/pnas.2412961121. Epub 2025 Jan 6. Proc Natl Acad Sci U S A. 2025. PMID: 39761402 Free PMC article.
-
[Homologs of MutS and MutL during mammalian meiosis].Med Sci (Paris). 2003 Jan;19(1):85-91. doi: 10.1051/medsci/200319185. Med Sci (Paris). 2003. PMID: 12836196 Review. French.
-
Factors underlying restricted crossover localization in barley meiosis.Annu Rev Genet. 2014;48:29-47. doi: 10.1146/annurev-genet-120213-092509. Epub 2014 Aug 1. Annu Rev Genet. 2014. PMID: 25089719 Review.
Cited by
-
Evolutionary mysteries in meiosis.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 19;371(1706):20160001. doi: 10.1098/rstb.2016.0001. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27619705 Free PMC article. Review.
-
Chromosome architecture and homologous recombination in meiosis.Front Cell Dev Biol. 2023 Jan 6;10:1097446. doi: 10.3389/fcell.2022.1097446. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684419 Free PMC article. Review.
-
Ovarian transcriptional response to Wolbachia infection in D. melanogaster in the context of between-genotype variation in gene expression.G3 (Bethesda). 2023 May 2;13(5):jkad047. doi: 10.1093/g3journal/jkad047. G3 (Bethesda). 2023. PMID: 36857313 Free PMC article.
-
Common and low-frequency variants associated with genome-wide recombination rate.Nat Genet. 2014 Jan;46(1):11-6. doi: 10.1038/ng.2833. Epub 2013 Nov 24. Nat Genet. 2014. PMID: 24270358
-
Two telomeric ends of acrocentric chromosome play distinct roles in homologous chromosome synapsis in the fetal mouse oocyte.Chromosoma. 2021 Mar;130(1):41-52. doi: 10.1007/s00412-021-00752-1. Epub 2021 Jan 25. Chromosoma. 2021. PMID: 33492414
References
-
- Hunter N. Meiotic recombination. In: Aguilera A, Rothstein R, editors. Molecular Genetics of Recombination. Springer-Verlag; Heidelberg, Germany: 2006. pp. 381–442.
-
- Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 1999;33:603–754. - PubMed
-
- Sakuno T, Tanaka K, Hauf S, Watanabe Y. Repositioning of aurora B promoted by chiasmata ensures sister chromatid mono-orientation in meiosis I. Dev. Cell. 2011;21:534–545. - PubMed
-
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum. Mol. Genet. 2007;16(Spec. No. 2):R203–R208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

