A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin
- PMID: 23396351
- PMCID: PMC3594569
- DOI: 10.1038/nsmb.2500
A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin
Abstract
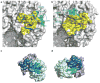
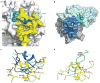
Influenza virus hemagglutinin (HA) mediates receptor binding and viral entry during influenza infection. The development of receptor analogs as viral-entry blockers has not been successful, which suggests that sialic acid may not be an ideal scaffold to obtain broad, potent HA inhibitors. Here, we report crystal structures of Fab fragments from three human antibodies that neutralize the 1957 pandemic H2N2 influenza virus in complex with H2 HA. All three antibodies use an aromatic residue to plug a conserved cavity in the HA receptor-binding site. Each antibody interacts with the absolutely conserved HA1 Trp153 at the cavity base through π-π stacking with the signature Phe54 of two VH1-69-encoded antibodies or a tyrosine from HCDR3 in the other antibody. This highly conserved interaction can be used as a starting point to design inhibitors targeting this conserved hydrophobic pocket in influenza viruses.
Conflict of interest statement
Vanderbilt University previously submitted a patent covering the diagnostic and therapeutic use of antibodies 2G1, 8M2 and 8F8 prior to the structural work described here.
Figures
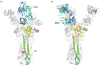


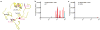

Comment in
-
Host versus flu: antibodies win a round?Nat Struct Mol Biol. 2013 Mar;20(3):245-6. doi: 10.1038/nsmb.2524. Nat Struct Mol Biol. 2013. PMID: 23463305 Free PMC article.
References
-
- Skehel J, Wiley D. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–69. - PubMed
-
- Paulson JC. The Receptors. Vol. 2. Academic Press; Orlando, FL: 1985. Interactions of animal viruses with cell surface receptors; pp. 131–219.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

