Epigenetic control of type 2 and 3 deiodinases in myogenesis: role of Lysine-specific Demethylase enzyme and FoxO3
- PMID: 23396445
- PMCID: PMC3616708
- DOI: 10.1093/nar/gkt065
Epigenetic control of type 2 and 3 deiodinases in myogenesis: role of Lysine-specific Demethylase enzyme and FoxO3
Abstract
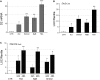
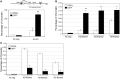
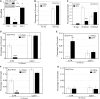
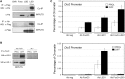
The proliferation and differentiation of muscle precursor cells require myogenic regulatory factors and chromatin modifiers whose concerted action dynamically regulates access to DNA and allows reprogramming of cells towards terminal differentiation. Type 2 deiodinase (D2), the thyroid hormone (TH)-activating enzyme, is sharply upregulated during myoblast differentiation, whereas type 3 deiodinase (D3), the TH-inactivating enzyme, is downregulated. The molecular determinants controlling synchronized D2 and D3 expression in muscle differentiation are completely unknown. Here, we report that the histone H3 demethylating enzyme (LSD-1) is essential for transcriptional induction of D2 and repression of D3. LSD-1 relieves the repressive marks (H3-K9me2-3) on the Dio2 promoter and the activation marks (H3-K4me2-3) on the Dio3 promoter. LSD-1 silencing impairs the D2 surge in skeletal muscle differentiation while inducing D3 expression thereby leading to a global decrease in intracellular TH production. Furthermore, endogenous LSD-1 interacts with FoxO3a, and abrogation of FoxO3-DNA binding compromises the ability of LSD-1 to induce D2. Our data reveal a novel epigenetic control of reciprocal deiodinases expression and provide a molecular mechanism by which LSD-1, through the opposite regulation of D2 and D3 expression, acts as a molecular switch that dynamically finely tunes the cellular needs of active TH during myogenesis.
Figures

References
-
- Sartorelli V, Puri PL. The link between chromatin structure, protein acetylation and cellular differentiation. Front Biosci. 2001;6:D1024–D1047. - PubMed
-
- Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005;16:585–595. - PubMed
-
- Forcales SV, Puri PL. Signaling to the chromatin during skeletal myogenesis: novel targets for pharmacological modulation of gene expression. Semin. Cell Dev. Biol. 2005;16:596–611. - PubMed
-
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

