Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis
- PMID: 23396504
- PMCID: PMC3586125
- DOI: 10.1136/bmjopen-2012-002360
Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis
Abstract
This review is an abridged version of a Cochrane Review previously published in the Cochrane Database of Systematic Reviews 2010, Issue 4, Art. No.: MR000013 DOI: 10.1002/14651858.MR000013.pub5 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
Objective: To identify interventions designed to improve recruitment to randomised controlled trials, and to quantify their effect on trial participation.
Design: Systematic review.
Data sources: The Cochrane Methodology Review Group Specialised Register in the Cochrane Library, MEDLINE, EMBASE, ERIC, Science Citation Index, Social Sciences Citation Index, C2-SPECTR, the National Research Register and PubMed. Most searches were undertaken up to 2010; no language restrictions were applied.
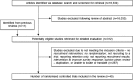
Study selection: Randomised and quasi-randomised controlled trials, including those recruiting to hypothetical studies. Studies on retention strategies, examining ways to increase questionnaire response or evaluating the use of incentives for clinicians were excluded. The study population included any potential trial participant (eg, patient, clinician and member of the public), or individual or group of individuals responsible for trial recruitment (eg, clinicians, researchers and recruitment sites). Two authors independently screened identified studies for eligibility.
Results: 45 trials with over 43 000 participants were included. Some interventions were effective in increasing recruitment: telephone reminders to non-respondents (risk ratio (RR) 1.66, 95% CI 1.03 to 2.46; two studies, 1058 participants), use of opt-out rather than opt-in procedures for contacting potential participants (RR 1.39, 95% CI 1.06 to 1.84; one study, 152 participants) and open designs where participants know which treatment they are receiving in the trial (RR 1.22, 95% CI 1.09 to 1.36; two studies, 4833 participants). However, the effect of many other strategies is less clear, including the use of video to provide trial information and interventions aimed at recruiters.
Conclusions: There are promising strategies for increasing recruitment to trials, but some methods, such as open-trial designs and opt-out strategies, must be considered carefully as their use may also present methodological or ethical challenges. Questions remain as to the applicability of results originating from hypothetical trials, including those relating to the use of monetary incentives, and there is a clear knowledge gap with regard to effective strategies aimed at recruiters.
Figures



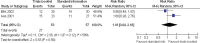
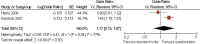
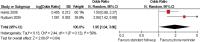
References
-
- Haidich AB, Ioannidis JPA. Patterns of patient enrollment in randomized controlled trials. J Clin Epidemiol 2001;54:877–83 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
