Comparison of routine prenatal iron prophylaxis and screening and treatment for anaemia: pregnancy results and preliminary birth results from a pragmatic randomised controlled trial (PROFEG) in Maputo, Mozambique
- PMID: 23396557
- PMCID: PMC3585968
- DOI: 10.1136/bmjopen-2012-001948
Comparison of routine prenatal iron prophylaxis and screening and treatment for anaemia: pregnancy results and preliminary birth results from a pragmatic randomised controlled trial (PROFEG) in Maputo, Mozambique
Abstract
Objective: To present the pregnancy results and interim birth results of a pragmatic randomised controlled trial comparing routine iron prophylaxis with screening and treatment for anaemia during pregnancy in a setting of endemic malaria and HIV.
Design: A pragmatic randomised controlled trial.
Setting: Two health centres (1° de Maio and Machava) in Maputo, Mozambique, a setting of endemic malaria and high prevalence of HIV.
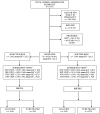
Participants: Pregnant women (≥18-year-olds; non-high-risk pregnancy, n=4326) attending prenatal care consultation at the two health centres were recruited to the trial.
Interventions: The women were randomly allocated to either Routine iron (n=2184; 60 mg ferrous sulfate plus 400 μg of folic acid daily throughout pregnancy) or Selective iron (n=2142; screening and treatment for anaemia and daily intake of 1 mg of folic acid).
Outcome measures: The primary outcomes were preterm delivery (delivery <37 weeks of gestation) and low birth weight (<2500 g). The secondary outcomes were symptoms suggestive of malaria and self-reported malaria during pregnancy; birth length; caesarean section; maternal and child health status after delivery.
Results: The number of follow-up visits was similar in the two groups. Between the first and fifth visits, the two groups were similar regarding the occurrence of fever, headache, cold/chills, nausea/vomiting and body aches. There was a suggestion of increased incidence of self-reported malaria during pregnancy (OR 1.37, 95% CI 0.98 to1.92) in the Routine iron group. Birth data were available for 1109 (51%) in the Routine iron group and for 1149 (54%) in the Selective iron group. The birth outcomes were relatively similar in the two groups. However, there was a suggestion (statistically non-significant) of poorer outcomes in the Routine iron group with regard to long hospital stay after birth (relative risk (RR) 1.43, 95% CI 0.97 to 1.26; risk difference (RD) 0.02, 95% CI -0.00 to 0.03) and unavailability of delivery data (RR 1.06, 95% CI 1.00 to 1.13; RD 0.03, 95% CI -0.01 to 0.07).
Conclusions: These interim results suggest that routine iron prophylaxis during pregnancy did not confer advantage over screening and treatment for anaemia regarding maternal and child health. Complete data on birth outcomes are being collected for firmer conclusions.
Trial registration: The trial is registered at ClinicalTrials.gov, number NCT00488579 (June 2007). The first women were randomised to the trial proper April 2007-March 2008. The pilot was November 2006-March 2008. The 3-month lag was due to technical difficulties in completing trial registration.
Figures
References
-
- Peña-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev 2009;(4):CD004736. - PubMed
-
- Villar J, Merialdi M, Gulmezoglu AM, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal mortality and preterm delivery: an overview of randomized controlled trials. J Nutr 2003;133:1606–25 - PubMed
-
- Lao TT, Tam KF, Chan LY. Third trimester iron status and pregnancy outcome in non-anaemic women: pregnancy unfavourably affected by maternal iron excess. Hum Reprod 2000;15:1843–8 - PubMed
-
- Yip R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: special consideration of iron nutrition. Am J Clin Nutr 2000;72:272S–9S - PubMed
-
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr 2001;131:616S–35S - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous