scFv-based "Grababody" as a general strategy to improve recruitment of immune effector cells to antibody-targeted tumors
- PMID: 23396586
- PMCID: PMC3630244
- DOI: 10.1158/0008-5472.CAN-12-3920
scFv-based "Grababody" as a general strategy to improve recruitment of immune effector cells to antibody-targeted tumors
Abstract
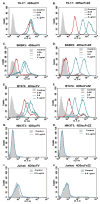
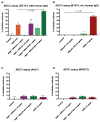
Recruitment of immune cells to tumor cells targeted by a therapeutic antibody can heighten the antitumor efficacy of the antibody. For example, p185(her2/neu)-targeting antibodies not only downregulate the p185(her2/neu) kinase (ERBB2) but also trigger complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) through the antibody Fc region. Here, we describe a generalized strategy to improve immune cell recruitment to targeted cancer cells, using a modified scFv antibody we call a "Grababody" that binds the target protein and endogenous immunoglobulins. The model system we used to illustrate the use of this platform recognizes p185(her2/neu) and includes an IgG binding domain. The recombinant scFv Grababody that was created recruited circulating human IgGs and attracted immune cells carrying Fc receptors to tumor cells that expressed p185(her2/neu). The presence of the IgG binding domain significantly enhanced CDC and ADCC activity and improved antitumor activity in vivo. Our results illustrate a novel general approach to improve antibody-like proteins for therapeutic applications.
©2013 AACR.
Conflict of interest statement
Figures

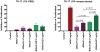

References
-
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature reviews Cancer. 2012;12:278–87. - PubMed
-
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. Journal of molecular biology. 2003;325:979–89. - PubMed
-
- Muthing J, Kemminer SE, Conradt HS, Sagi D, Nimtz M, Karst U, et al. Effects of buffering conditions and culture pH on production rates and glycosylation of clinical phase I anti-melanoma mouse IgG3 monoclonal antibody R24. Biotechnology and bioengineering. 2003;83:321–34. - PubMed
-
- Suzuki E, Niwa R, Saji S, Muta M, Hirose M, Iida S, et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:1875–82. - PubMed
-
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nature reviews Drug discovery. 2009;8:226–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

