Inhibition of human pancreatic tumor growth by cytokine-induced killer cells in nude mouse xenograft model
- PMID: 23396819
- PMCID: PMC3566419
- DOI: 10.4110/in.2012.12.6.247
Inhibition of human pancreatic tumor growth by cytokine-induced killer cells in nude mouse xenograft model
Abstract
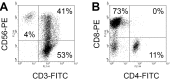
Pancreatic cancer is the fourth commonest cause of cancer-related deaths in the world. However, no adequate therapy for pancreatic cancer has yet been found. In this study, the antitumor activity of cytokine-induced killer (CIK) cells against the human pancreatic cancer was evaluated in vitro and in vivo. Human peripheral blood mononuclear cells were cultured with IL-2-containing medium in anti-CD3 for 14 days. The resulting populations of CIK cells comprised 94% CD3(+), 4% CD3(-)CD56(+), 41% CD3(+)CD56(+), 11% CD4(+), and 73% CD8(+). This heterogeneous cell population was called cytokine-induced killer (CIK) cells. At an effector-target cell ratio of 100:1, CIK cells destroyed 51% of AsPC-1 human pancreatic cancer cells, as measured by the (51)Cr-release assay. In addition, CIK cells at doses of 3 and 10 million cells per mouse inhibited 42% and 70% of AsPC-1 tumor growth in nude mouse xenograft assays, respectively. This study suggests that CIK cells may be used as an adoptive immunotherapy for pancreatic cancer patients.
Keywords: Adoptive immunotherapy; Cytokine-induced killer cells; Pancreatic cancer.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures



References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, Calderwood SK, Gong J. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2011;129:1990–2001. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

