Atypical protein kinase Cι is required for Wnt3a-dependent neurite outgrowth and binds to phosphorylated dishevelled 2
- PMID: 23396968
- PMCID: PMC3611013
- DOI: 10.1074/jbc.M112.448282
Atypical protein kinase Cι is required for Wnt3a-dependent neurite outgrowth and binds to phosphorylated dishevelled 2
Abstract
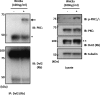
Previously we reported that Wnt3a-dependent neurite outgrowth in Ewing sarcoma family tumor cell lines was mediated by Frizzled3, Dishevelled (Dvl), and c-Jun N-terminal kinase (Endo, Y., Beauchamp, E., Woods, D., Taylor, W. G., Toretsky, J. A., Uren, A., and Rubin, J. S. (2008) Mol. Cell. Biol. 28, 2368-2379). Subsequently, we observed that Dvl2/3 phosphorylation correlated with neurite outgrowth and that casein kinase 1δ, one of the enzymes that mediate Wnt3a-dependent Dvl phosphorylation, was required for neurite extension (Greer, Y. E., and Rubin, J. S. (2011) J. Cell Biol. 192, 993-1004). However, the functional relevance of Dvl phosphorylation in neurite outgrowth was not established. Dvl1 has been shown by others to be important for axon specification in hippocampal neurons via an interaction with atypical PKCζ, but the role of Dvl phosphorylation was not evaluated. Here we report that Ewing sarcoma family tumor cells express PKCι but not PKCζ. Wnt3a stimulated PKCι activation and caused a punctate distribution of pPKCι in the neurites and cytoplasm, with a particularly intense signal at the centrosome. Knockdown of PKCι expression with siRNA reagents blocked neurite formation in response to Wnt3a. Aurothiomalate, a specific inhibitor of PKCι/Par6 binding, also suppressed neurite extension. Wnt3a enhanced the co-immunoprecipitation of endogenous PKCι and Dvl2. Although FLAG-tagged wild-type Dvl2 immunoprecipitated with PKCι, a phosphorylation-deficient Dvl2 derivative did not. This derivative also was unable to rescue neurite outgrowth when endogenous Dvl2/3 was suppressed by siRNA (González-Sancho, J. M., Greer, Y. E., Abrahams, C. L., Takigawa, Y., Baljinnyam, B., Lee, K. H., Lee, K. S., Rubin, J. S., and Brown, A. M. (2013) J. Biol. Chem. 288, 9428-9437). Taken together, these results suggest that site-specific Dvl2 phosphorylation is required for Dvl2 association with PKCι. This interaction is likely to be one of the mechanisms essential for Wnt3a-dependent neurite outgrowth.
Figures






References
-
- Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 - PubMed
-
- Malaterre J., Ramsay R. G., Mantamadiotis T. (2007) Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front. Biosci. 12, 492–506 - PubMed
-
- Montcouquiol M., Crenshaw E. B., 3rd, Kelley M. W. (2006) Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29, 363–386 - PubMed
-
- Bovolenta P., Rodriguez J., Esteve P. (2006) Frizzled/RYK mediated signalling in axon guidance. Development 133, 4399–4408 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

