Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation
- PMID: 23396975
- PMCID: PMC3622323
- DOI: 10.1194/jlr.M032862
Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation
Abstract
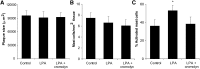
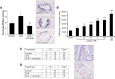
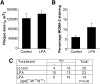
Lysophosphatidic acid (LPA), a bioactive lysophospholipid, accumulates in the atherosclerotic plaque. It has the capacity to activate mast cells, which potentially exacerbates plaque progression. In this study, we thus aimed to investigate whether LPA contributes to plaque destabilization by modulating mast cell function. We here show by an imaging mass spectrometry approach that several LPA species are present in atherosclerotic plaques. Subsequently, we demonstrate that LPA is a potent mast cell activator which, unlike other triggers, favors release of tryptase. Local perivascular administration of LPA to an atherosclerotic carotid artery segment increases the activation status of perivascular mast cells and promotes intraplaque hemorrhage and macrophage recruitment without impacting plaque cell apoptosis. The mast cell stabilizer cromolyn could prevent intraplaque hemorrhage elicited by LPA-mediated mast cell activation. Finally, the involvement of mast cells in these events was further emphasized by the lack of effect of perivascular LPA administration in mast cell deficient animals. We demonstrate that increased accumulation of LPA in plaques induces perivascular mast cell activation and in this way contributes to plaque destabilization in vivo. This study points to local LPA availability as an important factor in atherosclerotic plaque stability.
Figures


Comment in
-
Lysophosphatidic acid and cardiovascular disease: seeing is believing.J Lipid Res. 2013 May;54(5):1153-5. doi: 10.1194/jlr.E037887. Epub 2013 Mar 18. J Lipid Res. 2013. PMID: 23509404 Free PMC article. No abstract available.
References
-
- Shah P. K. 2003. Mechanisms of plaque vulnerability and rupture. J. Am. Coll. Cardiol. 41: 15S–22S - PubMed
-
- Libby P. 2002. Inflammation in atherosclerosis. Nature. 420: 868–874 - PubMed
-
- Weber C., Zernecke A., Libby P. 2008. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8: 802–815 - PubMed
-
- Kaartinen M., Pentillä A., Kovanen P. T. 1994. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 90: 1669–1678 - PubMed
-
- Laine P., Kaartinen M., Pentillä A., Panula P., Paavonen T., Kovanen P. T. 1999. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 99: 361–369 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

