Developmental mechanisms directing early anterior forebrain specification in vertebrates
- PMID: 23397132
- PMCID: PMC3781296
- DOI: 10.1007/s00018-013-1269-5
Developmental mechanisms directing early anterior forebrain specification in vertebrates
Abstract
Research from the last 15 years has provided a working model for how the anterior forebrain is induced and specified during the early stages of embryogenesis. This model relies on three basic processes: (1) induction of the neural plate from naive ectoderm requires the inhibition of BMP/TGFβ signaling; (2) induced neural tissue initially acquires an anterior identity (i.e., anterior forebrain); (3) maintenance and expansion of the anterior forebrain depends on the antagonism of posteriorizing signals that would otherwise transform this tissue into posterior neural fates. In this review, we present a historical perspective examining some of the significant experiments that have helped to delineate this molecular model. In addition, we discuss the function of the relevant tissues that act prior to and during gastrulation to ensure proper anterior forebrain formation. Finally, we elaborate data, mainly obtained from the analyses of mouse mutants, supporting a role for transcriptional repressors in the regulation of cell competence within the anterior forebrain. The aim of this review is to provide the reader with a general overview of the signals as well as the signaling centers that control the development of the anterior neural plate.
Figures
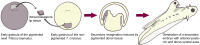
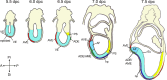


References
-
- Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, Simeone A. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125(24):5091–5104. - PubMed
-
- Albazerchi A, Stern CD. A role for the hypoblast (AVE) in the initiation of neural induction, independent of its ability to position the primitive streak. Dev Biol. 2007;301(2):489–503. - PubMed
-
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78(4):561–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

