Characterization of distinct single-channel properties of Ca²⁺ inward currents in mitochondria
- PMID: 23397170
- PMCID: PMC3696464
- DOI: 10.1007/s00424-013-1224-1
Characterization of distinct single-channel properties of Ca²⁺ inward currents in mitochondria
Abstract
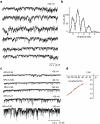
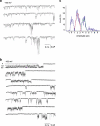
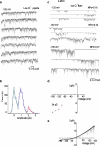
Previous studies have demonstrated several molecularly distinct players involved in mitochondrial Ca(2+) uptake. In the present study, electrophysiological recordings on mitoplasts that were isolated from HeLa cells were performed in order to biophysically and pharmacologically characterize Ca(2+) currents across the inner mitochondrial membrane. In mitoplast-attached configuration with 105 mM Ca(2+) as a charge carrier, three distinct channel conductances of 11, 23, and 80 pS were observed. All types of mitochondrial currents were voltage-dependent and essentially depended on the presence of Ca(2+) in the pipette. The 23 pS channel exhibited burst kinetics. Though all channels were sensitive to ruthenium red, their sensitivity was different. The 11 and 23 pS channels exhibited a lower sensitivity to ruthenium red than the 80 pS channel. The activities of all channels persisted in the presence of cylosporin A, CGP 37187, various K(+)-channel inhibitors, and Cl(-) channel blockers disodium 4,4'-diisothiocyanatostilbene-2,2'-disulfonate and niflumic acid. Collectively, our data identified multiple conductances of Ca(2+) currents in mitoplasts isolated from HeLa cells, thus challenging the dogma of only one unique mitochondrial Ca(2+) uniporter.
Figures







References
-
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. - DOI - PubMed
-
- Bahamonde MI, Valverde MA. Voltage-dependent anion channel localises to the plasma membrane and peripheral but not perinuclear mitochondria. Pflugers Arch. 2003;446:309–313. - PubMed
-
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. - DOI - PMC - PubMed
-
- Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–2939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

