ESR statement on the stepwise development of imaging biomarkers
- PMID: 23397519
- PMCID: PMC3609959
- DOI: 10.1007/s13244-013-0220-5
ESR statement on the stepwise development of imaging biomarkers
Abstract
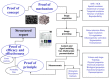
Development of imaging biomarkers is a structured process in which new biomarkers are discovered, verified, validated and qualified against biological processes and clinical end-points. The validation process not only concerns the determination of the sensitivity and specificity but also the measurement of reproducibility. Reproducibility assessments and standardisation of the acquisition and data analysis methods are crucial when imaging biomarkers are used in multicentre trials for assessing response to treatment. Quality control in multicentre trials can be performed with the use of imaging phantoms. The cost-effectiveness of imaging biomarkers also needs to be determined. A lot of imaging biomarkers are being developed, but there are still unmet needs-for example, in the detection of tumour invasiveness. Main Messages • Using imaging biomarkers to streamline drug discovery and disease progression is a huge advancement in healthcare. • The qualification and technical validation of imaging biomarkers pose unique challenges in that the accuracy, methods, standardisations and reproducibility are strictly monitored. • The clinical value of new biomarkers is of the highest priority in terms of patient management, assessing risk factors and disease prognosis.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous