Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling
- PMID: 23397885
- PMCID: PMC3797455
- DOI: 10.1089/ars.2012.4599
Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling
Abstract
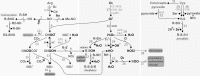
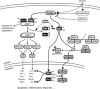
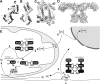
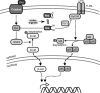
Thioredoxins (Trxs), glutaredoxins (Grxs), and peroxiredoxins (Prxs) have been characterized as electron donors, guards of the intracellular redox state, and "antioxidants". Today, these redox catalysts are increasingly recognized for their specific role in redox signaling. The number of publications published on the functions of these proteins continues to increase exponentially. The field is experiencing an exciting transformation, from looking at a general redox homeostasis and the pathological oxidative stress model to realizing redox changes as a part of localized, rapid, specific, and reversible redox-regulated signaling events. This review summarizes the almost 50 years of research on these proteins, focusing primarily on data from vertebrates and mammals. The role of Trx fold proteins in redox signaling is discussed by looking at reaction mechanisms, reversible oxidative post-translational modifications of proteins, and characterized interaction partners. On the basis of this analysis, the specific regulatory functions are exemplified for the cellular processes of apoptosis, proliferation, and iron metabolism. The importance of Trxs, Grxs, and Prxs for human health is addressed in the second part of this review, that is, their potential impact and functions in different cell types, tissues, and various pathological conditions.
Figures



References
-
- Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. - PubMed
-
- Abdiu A. Nakamura H. Sahaf B. Yodoi J. Holmgren A. Rosén A. Thioredoxin blood level increases after severe burn injury. Antioxid Redox Signal. 2000;2:707–716. - PubMed
-
- Adlard PA. Bush AI. Metals and Alzheimer's disease. J Alzheimers Dis. 2006;10:145–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

