A controlled study on baby-friendly communities in Italy: methods and baseline data
- PMID: 23398142
- PMCID: PMC3616411
- DOI: 10.1089/bfm.2012.0130
A controlled study on baby-friendly communities in Italy: methods and baseline data
Abstract
Aim: This study reports the research methods and baseline data of a project aimed at assessing the effect of an intervention based on the 7 Steps of the Baby Friendly Community Initiative (BFCI) on the rate of exclusive breastfeeding at 6 months in Italy.
Subjects and methods: In this controlled, nonrandomized study, nine Local Health Authorities were assigned to an early and nine to a late intervention group. Data on breastfeeding in infants followed up from birth to 12 months were gathered at baseline and in two subsequent rounds, after the 7 Steps were implemented in the early and late intervention groups, respectively. Step-down logistic regression analysis, corrected for the cluster effect, was used to compare breastfeeding rates between groups.
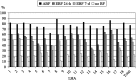
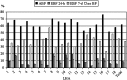
Results: At baseline, there were no significant differences in breastfeeding rates at birth (n=1,781) and at 3 (n=1,854), 6 (n=1,601), and 12 (n=1,510; loss to follow-up, 15.2%) months between groups. At birth, 96% of mothers initiated breastfeeding, 72% exclusively (recall from birth). At 3 months, 77% of infants were breastfed, 54% exclusively with 24-hour and 46% with 7-day recall. At 6 months, the rate of any breastfeeding was 62%, with 10% and 7% exclusive breastfeeding with 24-hour and 7-day recall, respectively. At 12 months, 31% of the children continued to breastfeed.
Conclusions: The project is ongoing and will allow estimation of the effect of the BFCI.
Figures


References
-
- Kramer MS. Chalmers B. Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA. 2001;285:413–420. - PubMed
-
- World Health Organization. World Health Organization; Geneva: 2002. Global Strategy for Infant and Young Child Feeding.
-
- Ministry of Health; Gazzetta Ufficiale, Numero 32. Ministero della Salute; Rome: Feb 7, 2008. Linee di indirizzo nazionali sulla protezione, la promozione ed il sostegno dell'allattamento al seno.
-
- International Lactation Consultant Association. International Lactation Consultant Association; Raleigh, NC: 2000. Position Paper on Infant Feeding.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

