Reconstitution of the myocardium in regenerating newt hearts is preceded by transient deposition of extracellular matrix components
- PMID: 23398466
- PMCID: PMC3685393
- DOI: 10.1089/scd.2012.0575
Reconstitution of the myocardium in regenerating newt hearts is preceded by transient deposition of extracellular matrix components
Abstract
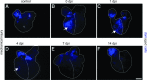
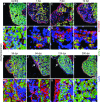
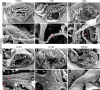
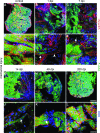
Adult newts efficiently regenerate the heart after injury in a process that involves proliferation of cardiac muscle and nonmuscle cells and repatterning of the myocardium. To analyze the processes that underlie heart regeneration in newts, we characterized the structural changes in the myocardium that allow regeneration after mechanical injury. We found that cardiomyocytes in the damaged ventricle mainly die by necrosis and are removed during the first week after injury, paving the way for the extension of thin myocardial trabeculae, which initially contain only very few cardiomyocytes. During the following 200 days, these thin trabeculae fill up with new cardiomyocytes until the myocardium is fully reconstituted. Interestingly, reconstruction of the newly formed trabeculated network is accompanied by transient deposition of extracellular matrix (ECM) components such as collagen III. We conclude that the ECM is a critical guidance cue for outgrowing and branching trabeculae to reconstruct the trabeculated network, which represents a hallmark of uninjured cardiac tissue in newts.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

