Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT)
- PMID: 23399084
- PMCID: PMC4698888
- DOI: 10.1111/cts.12003
Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT)
Abstract
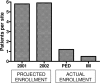
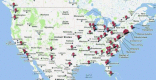
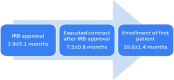
We describe the experience of the focal segmental glomerulosclerosis clinical trial (FSGS CT) in the identification and recruitment of participants into the study. This National Institutes of Health funded study, a multicenter, open-label, randomized comparison of cyclosporine versus oral dexamethasone pulses plus mycophenolate mofetil, experienced difficulty and delays meeting enrollment goals. These problems occurred despite the support of patient advocacy groups and aggressive recruitment strategies. Multiple barriers were identified including: (1) inaccurate estimates of the number of potential incident FSGS patients at participating centers; (2) delays in securing one of the test agents; (3) prolonged time between IRB approval and execution of a subcontract (mean 7.5 ± 0.8 months); (4) prolonged time between IRB approval and enrollment of the first patient at participating sites (mean 19.6 ± 1.4 months); and (5) reorganization of clinical coordinating core infrastructure to align resources with enrollment. A Web-based anonymous survey of site investigators revealed site-related barriers to patient recruitment. The value of a variety of recruitment tools was of marginal utility in facilitating patient enrollment. We conclude that improvements in the logistics of study approval and regulatory start-up and testing of promising novel agents are important factors in promoting enrollment into randomized clinical trials in nephrology.
© 2013 Wiley Periodicals, Inc.
Figures
References
-
- Strippoli GF, Craig JC, Schena FP. The number, quality and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol. 2004; 15: 411–419. - PubMed
-
- Johnston SC, Rootenberg JD, Katrak S, Smith WS, Elkins JS. Effect of a US National Institutes of Health programme of clinical trials on public health and costs. Lancet 2006; 367(9519): 1319–1327. doi:. PMID 16631910. http://www.chrp.org/pdf/HSR20070511.pdf. Available at: http://www.usrds.org/2011/view/v2_11.asp. Accessed February 2, 2012. - DOI - PubMed
-
- Deegens JKJ, Wetzels JFM. Immunosuppressive treatment of focal segmental glomerulosclerosis: lessons from a randomized controlled trial. Kidney Int. 2011; 80: 798–801. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 DK070341/DK/NIDDK NIH HHS/United States
- DK063549/DK/NIDDK NIH HHS/United States
- DK063455/DK/NIDDK NIH HHS/United States
- U01 DK063455/DK/NIDDK NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- U01 DK063490/DK/NIDDK NIH HHS/United States
- UL1 RR0249860/RR/NCRR NIH HHS/United States
- UL1 RR025750/RR/NCRR NIH HHS/United States
- UL1 RR05758/RR/NCRR NIH HHS/United States
- U01 DK063549/DK/NIDDK NIH HHS/United States
- U01 DK063385/DK/NIDDK NIH HHS/United States
- UL1 RR029882/RR/NCRR NIH HHS/United States
- U01-DK063385/DK/NIDDK NIH HHS/United States
- DK063490/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

