Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior
- PMID: 23399323
- PMCID: PMC3633643
- DOI: 10.1016/j.yhbeh.2013.02.001
Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior
Abstract
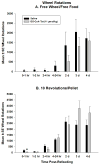
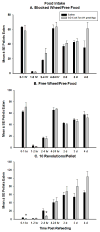
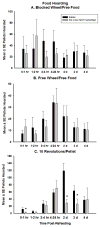
Ghrelin is an orexigenic hormone produced by the stomach in direct proportion to the time since the last meal and has therefore been called a 'hunger signal'. The octanoylation of ghrelin is critical for its orexigenic functions and is dependent upon ghrelin O-acyltransferase (GOAT) catalyzation. The GOAT inhibitor, GO-CoA-Tat, decreases the circulating concentrations of octanoylated ghrelin and attenuates weight gain on a high fat diet in mice. Unlike rats and mice, Siberian hamsters and humans do not increase food intake after food deprivation, but increase food hoarding after food deprivation. In Siberian hamsters, exogenous ghrelin increases ingestive behaviors similarly to 48-56 h food deprivation. Therefore, we tested the necessity of increased ghrelin in food-deprived Siberian hamsters to stimulate ingestive behaviors. To do so we used our simulated natural housing system that allows hamsters to forage for and hoard food. Animals were given an injection of GO-CoA-Tat (i.p., 11 μmol/kg) every 6h because that is the duration of its effective inhibition of octanoylated ghrelin concentrations during a 48 h food deprivation. We found that GO-CoA-Tat attenuated food foraging (0-1h), food intake (0-1 and 2-4h), and food hoarding (0-1h and 2 and 3 days) post-refeeding compared with saline treated animals. This suggests that increased octanoylated ghrelin concentrations play a role in the food deprivation-induced increases in ingestive behavior. Therefore, ghrelin is a critical aspect of the multi-faceted mechanisms that stimulate ingestive behaviors, and might be a critical point for a successful clinical intervention scheme in humans.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
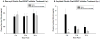
References
-
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. - PMC - PubMed
-
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. - PubMed
-
- Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschop MH, Boeke JD, Cole PA. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

