Comparison of immune response by virus infection and vaccination to 2009 pandemic influenza A/H1N1 in children
- PMID: 23399558
- PMCID: PMC3565140
- DOI: 10.3346/jkms.2013.28.2.274
Comparison of immune response by virus infection and vaccination to 2009 pandemic influenza A/H1N1 in children
Abstract
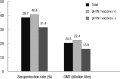
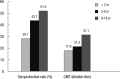
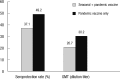
We aimed to compare the immune response induced by natural infection with 2009 pandemic influenza A/H1N1 (pH1N1) virus and by monovalent pH1N1 vaccination in children and adolescents. This cross-sectional clinical study was conducted at 3 hospitals in Korea from February to May 2010. A total of 266 healthy subjects aged from 6 months to 18 yr were tested for the presence of the antibody against pH1N1 using hemagglutination inhibition (HI) test. Information about pH1N1 vaccination and laboratory-confirmed pH1N1 infection history was obtained. The overall rate of HI titers of ≥ 1:40 against pH1N1 was 38.7%, and the geometric mean titer (GMT) was 20.5. Immunogenicity of pH1N1 vaccination only was reflected by a 41.1% of seroprotection rate and a GMT of 22.5. Immunogenicity of natural infection only was reflected by a 61.0% of seroprotection rate and a GMT of 40.0. GMT was significantly higher in the subjects of natural infection group than in the subjects of pH1N1 vaccination group (P < 0.001). The immune responses induced by natural pH1N1 infection exceed those induced by pH1N1 vaccinations.
Keywords: Child; Immune Response; Natural Infection; Pandemic Influenza A; Vaccination.
Figures
References
-
- CDC. Swine influenza A (H1N1) infection in two children-Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:400–402. - PubMed
-
- CDC. Outbreak of swine origin influenza A (H1N1) virus infection-Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–470. - PubMed
-
- Kreijtz JH, Fouchier RA, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011;162:19–30. - PubMed
-
- Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000;18:957–1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical