Lumican expression, localization and antitumor activity in prostate cancer
- PMID: 23399832
- PMCID: PMC3633477
- DOI: 10.1016/j.yexcr.2013.01.023
Lumican expression, localization and antitumor activity in prostate cancer
Abstract
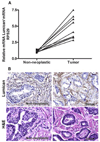
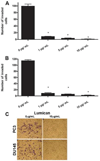
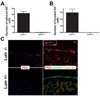
The stromal reaction surrounding tumors leads to the formation of a tumor-specific microenvironment, which may play either a restrictive role or a supportive role in the growth and progression of the tumors. Lumican, a small leucine-rich proteoglycan (SLRP) of the extracellular matrix (ECM), regulates collagen fibrillogenesis. Recently, lumican has also been shown to regulate cell behavior during embryonic development, tissue repair and tumor progression. The role of lumican in cancer varies according to the type of tumor. In this study we analyze the role of lumican in the pathogenesis of prostate cancer both in vivo and in vitro. Overall lumican up-regulation was observed in the primary tumors analyzed through both real-time PCR and immunostaining. The increase in lumican expression was observed in the reactive stroma surrounding prostate primary tumors with fibrotic deposition surrounding the acinar glands. In vitro analysis demonstrated that lumican inhibited both the migration and invasion of metastatic prostate cancer cells isolated from lymph node, bone and brain. Moreover, prostate cancer cells seeded on lumican presented a decrease in the formation of cellular projections, lamellipodia detected by a decreased rearrangement in ZO-1, keratin 8/18, integrin β1 and MT1-MMP, and invadopodia detected by disruption of α-smooth muscle actin, cortactin and N-WASP. Moreover, a significant increase in prostate cancer cell invasion was observed through the peritoneum of lumican knockout mice, further demonstrating the restrictive role lumican present in the ECM has on prostate cancer invasion. In conclusion, lumican present in the reactive stroma surrounding prostate primary tumors plays a restrictive role on cancer progression, and we therefore postulate that lumican could be a valuable marker in prostate cancer staging.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Effects of the extracellular matrix on lumican expression in rat aortic smooth muscle cells in vitro.J Pathol. 2001 Dec;195(5):604-8. doi: 10.1002/path.994. J Pathol. 2001. PMID: 11745697
-
Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion complexes.Cancer Lett. 2009 Sep 28;283(1):92-100. doi: 10.1016/j.canlet.2009.03.032. Epub 2009 Apr 24. Cancer Lett. 2009. PMID: 19394140
-
Different expression levels of lumican in human carcinoid tumor and neuroendocrine cell carcinoma.Int J Oncol. 2005 Apr;26(4):873-80. Int J Oncol. 2005. PMID: 15753980
-
Functions of lumican and fibromodulin: lessons from knockout mice.Glycoconj J. 2002 May-Jun;19(4-5):287-93. doi: 10.1023/A:1025348417078. Glycoconj J. 2002. PMID: 12975607 Review.
-
Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth.J Nippon Med Sch. 2005 Jun;72(3):137-45. doi: 10.1272/jnms.72.137. J Nippon Med Sch. 2005. PMID: 16046829 Review.
Cited by
-
Angiostatic cues from the matrix: Endothelial cell autophagy meets hyaluronan biology.J Biol Chem. 2020 Dec 4;295(49):16797-16812. doi: 10.1074/jbc.REV120.014391. Epub 2020 Oct 5. J Biol Chem. 2020. PMID: 33020183 Free PMC article. Review.
-
Lumican in Carcinogenesis-Revisited.Biomolecules. 2021 Sep 6;11(9):1319. doi: 10.3390/biom11091319. Biomolecules. 2021. PMID: 34572532 Free PMC article. Review.
-
Analysis of Cancer Stromal Reaction Using an O-ring Co-culture Assay.Bio Protoc. 2017 Feb 20;7(4):e2131. doi: 10.21769/BioProtoc.2131. eCollection 2017 Feb 20. Bio Protoc. 2017. PMID: 34458452 Free PMC article.
-
Identifying new biomarkers of aggressive Group 3 and SHH medulloblastoma using 3D hydrogel models, single cell RNA sequencing and 3D OrbiSIMS imaging.Acta Neuropathol Commun. 2023 Jan 11;11(1):6. doi: 10.1186/s40478-022-01496-4. Acta Neuropathol Commun. 2023. PMID: 36631900 Free PMC article.
-
Lumican mediates HTB94 chondrosarcoma cell growth via an IGF‑IR/Erk1/2 axis.Int J Oncol. 2020 Sep;57(3):791-803. doi: 10.3892/ijo.2020.5094. Epub 2020 Jul 6. Int J Oncol. 2020. PMID: 32705211 Free PMC article.
References
-
- Proia DA, Kuperwasser C. Stroma: tumor agonist or antagonist. Cell Cycle. 2005;4:1022–1025. - PubMed
-
- Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, de Paula CA, Mader AM, Waisberg J, Pinhal MA, Friedl A, Toma L, Nader HB. Colorectal cancer desmoplastic reaction up-regulates collagen synthesis and restricts cancer cell invasion. Cell Tissue Res. 2011;346:223–236. - PubMed
-
- Coulson-Thomas VJ, Gesteira TF, Coulson-Thomas YM, Vicente CM, Tersariol IL, Nader HB, Toma L. Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix down-regulation. Exp Cell Res. 2010;316:3207–3226. - PubMed
-
- Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

