Gads (Grb2-related adaptor downstream of Shc) is required for BCR-ABL-mediated lymphoid leukemia
- PMID: 23399893
- PMCID: PMC4981500
- DOI: 10.1038/leu.2013.40
Gads (Grb2-related adaptor downstream of Shc) is required for BCR-ABL-mediated lymphoid leukemia
Abstract
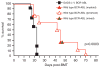
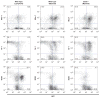
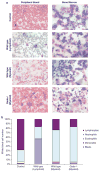
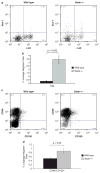
Philadelphia chromosome-positive leukemias, including chronic myeloid leukemia and B-cell acute lymphoblastic leukemia (B-ALL), are driven by the oncogenic BCR-ABL fusion protein. Animal modeling experiments utilizing retroviral transduction and subsequent bone marrow transplantation have demonstrated that BCR-ABL generates both myeloid and lymphoid disease in mice receiving whole bone marrow transduced with BCR-ABL. Y177 of BCR-ABL is critical to the development of myeloid disease, and phosphorylation of Y177 has been shown to induce GRB2 binding to BCR-ABL, followed by activation of the Ras and phosphoinositide 3 kinase signaling pathways. We show that the GRB2-related adapter protein, GADS, also associates with BCR-ABL, specifically through Y177 and demonstrate that BCR-ABL-driven lymphoid disease requires Gads. BCR-ABL transduction of Gads(-/-) bone marrow results in short latency myeloid disease within 3-4 weeks of transplant, while wild-type mice succumb to both a longer latency lymphoid and myeloid diseases. We report that GADS mediates a unique BCR-ABL complex with SLP-76 in BCR-ABL-positive cell lines and B-ALL patient samples. These data suggest that GADS mediates lymphoid disease downstream of BCR-ABL through the recruitment of specific signaling intermediates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. - PubMed
-
- Rowley JD. Chromosomal patterns in myelocytic leukemia. N Engl J Med. 1973;289:220–221. - PubMed
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. - PubMed
-
- O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012;12:513–526. - PubMed
-
- Konopka JB, Watanabe SM, Witte ON. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984;37:1035–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

