An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors
- PMID: 23399917
- PMCID: PMC3783638
- DOI: 10.1038/mp.2013.12
An important role for cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors
Erratum in
- Mol Psychiatry. 2014 Mar;19(3):400
Abstract
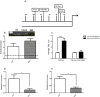
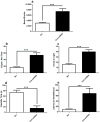
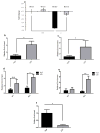
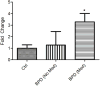
Mice with a mutation in the Clock gene (ClockΔ19) have been identified as a model of mania; however, the mechanisms that underlie this phenotype, and the changes in the brain that are necessary for lithium's effectiveness on these mice remain unclear. Here, we find that cholecystokinin (Cck) is a direct transcriptional target of CLOCK and levels of Cck are reduced in the ventral tegmental area (VTA) of ClockΔ19 mice. Selective knockdown of Cck expression via RNA interference in the VTA of wild-type mice produces a manic-like phenotype. Moreover, chronic treatment with lithium restores Cck expression to near wild-type and this increase is necessary for the therapeutic actions of lithium. The decrease in Cck expression in the ClockΔ19 mice appears to be due to a lack of interaction with the histone methyltransferase, MLL1, resulting in decreased histone H3K4me3 and gene transcription, an effect reversed by lithium. Human postmortem tissue from bipolar subjects reveals a similar increase in Cck expression in the VTA with mood stabilizer treatment. These studies identify a key role for Cck in the development and treatment of mania, and describe some of the molecular mechanisms by which lithium may act as an effective antimanic agent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kupfer DJ, Angst J, Berk M, Dickerson F, Frangou S, Frank E, et al. Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar Disorder. Ann N Y Acad Sci. 2011;1242(1):1–25. - PubMed
-
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–277. Spec No 2. - PubMed
-
- Lamont EW, Coutu DL, Cermakian N, Boivin DB. Circadian rhythms and clock genes in psychotic disorders. Isr J Psychiatry Relat Sci. 2010;47(1):27–35. - PubMed
-
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):631–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

