Detection of impaired homologous recombination repair in NSCLC cells and tissues
- PMID: 23399959
- PMCID: PMC3573529
- DOI: 10.1097/JTO.0b013e31827ecf83
Detection of impaired homologous recombination repair in NSCLC cells and tissues
Abstract
Introduction: Homologous recombination repair (HRR) is a critical pathway for the repair of DNA damage caused by cisplatin or poly-ADP ribose polymerase (PARP) inhibitors. HRR may be impaired by multiple mechanisms in cancer, which complicates assessing the functional HRR status in cells. Here, we monitored the ability of non-small-cell lung cancer (NSCLC) cells to form subnuclear foci of DNA repair proteins as a surrogate of HRR proficiency.
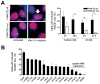
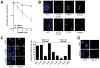
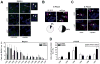
Methods: We assessed clonogenic survival of 16 NSCLC cell lines in response to cisplatin, mitomycin C (MMC), and the PARP inhibitor olaparib. Thirteen tumor explants from patients with NSCLC were subjected to cisplatin ex vivo. Cells were assayed for foci of repair-associated proteins such as BRCA1, FANCD2, RAD51, and γ-H2AX.
Results: Four cell lines (25%) showed an impaired RAD51 foci-forming ability in response to cisplatin. Impaired foci formation correlated with cellular sensitivity to cisplatin, MMC and olaparib. Foci responses complemented or superseded genomic information suggesting alterations in the ATM/ATR and FA/BRCA pathways. Because baseline foci in untreated cells did not predict drug sensitivity, we adapted an ex vivo biomarker assay to monitor damage-induced RAD51 foci in NSCLC explants from patients. Ex vivo cisplatin treatment of explants identified two tumors (15%) exhibiting compromised RAD51 foci induction.
Conclusions: A fraction of NSCLC harbors HRR defects that may sensitize the affected tumors to DNA-damaging agents including PARP inhibitors. We propose that foci-based functional biomarker assays represent a powerful tool for prospective determination of treatment sensitivity, but will require ex vivo techniques for induction of DNA damage to unmask the underlying HRR defect.
Conflict of interest statement
Figures


References
-
- Shepherd FA, Rosell R. Weighing tumor biology in treatment decisions for patients with non-small cell lung cancer. J Thorac Oncol. 2007;2 (Suppl 2):S68–76. - PubMed
-
- Willers H, Pfäffle HN, Zou L. Targeting Homologous Recombination Repair in Cancer. Academic Press, Elsevier; 2012. pp. 119–160.
-
- Ferrer M, Span SW, Vischioni B, Oudejans JJ, van Diest PJ, de Winter JP, Giaccone G, Kruyt FA. FANCD2 expression in advanced non-small-cell lung cancer and response to platinum-based chemotherapy. Clin Lung Cancer. 2005;6:250–254. - PubMed
-
- Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT. Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004;23:1000–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

