Expression pattern implicates a potential role for luman recruitment factor in the process of implantation in uteri and development of preimplantation embryos in mice
- PMID: 23400243
- PMCID: PMC3934142
- DOI: 10.1262/jrd.2012-137
Expression pattern implicates a potential role for luman recruitment factor in the process of implantation in uteri and development of preimplantation embryos in mice
Abstract
Luman/CREB3 recruitment factor (LRF or CREBRF) was identified as a regulator of Luman (or CREB3) that is involved in the unfolded protein response during endoplasmic reticulum stress. Luman is implicated in a multitude of functions ranging from viral infection and immunity to cancer. The biological function of LRF, however, is unknown. In this paper, we report that uteri of pregnant mice and embryos displayed enhanced LRF expression at all stages, and the expressed LRF was found to be localized specifically at implantation sites. On the other hand, uteri of mice induced for delayed implantation or pseudopregnant mice showed low levels of LRF expression, suggesting that LRF mediates uterine receptivity during implantation. Further, expression of LRF was found to be modulated by steroid hormones such as progesterone and estradiol. This study thereby identifies a potential role for LRF in the process of implantation in uteri and development of preimplantation embryos in mice.
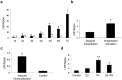
Figures


References
-
- Dey SK, Lim H, Das SK, Reese J, Paria B, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004; 25: 341–373 - PubMed
-
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nature Reviews Genetics 2006; 7: 185–199 - PubMed
-
- Kimber SJ, Spanswick C. Blastocyst implantation: the adhesion cascade. Semin Cell Dev Biol 2000; 11: 77–92 - PubMed
-
- Lee KY, DeMayo FJ. Animal models of implantation. Reproduction 2004; 128: 679–695 - PubMed
-
- Yoshinaga K. Review of factors essential for blastocyst implantation for their modulating effects on the maternal immune system. Semin Cell Dev Biol 2008; 19: 161–169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

