Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study
- PMID: 23400715
- PMCID: PMC3654174
- DOI: 10.1002/art.37824
Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study
Abstract
Objective: To evaluate the safety and tolerability of multiple intravenous (IV) doses of sifalimumab in adults with moderate-to-severe systemic lupus erythematosus (SLE).
Methods: In this multicenter, double-blind, placebo-controlled, sequential dose-escalation study, patients were randomized 3:1 to receive IV sifalimumab (0.3, 1.0, 3.0, or 10.0 mg/kg) or placebo every 2 weeks to week 26, then followed up for 24 weeks. Safety assessment included recording of treatment-emergent adverse events (AEs) and serious AEs. Pharmacokinetics, immunogenicity, and pharmacodynamics were evaluated, and disease activity was assessed.
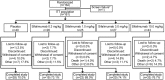
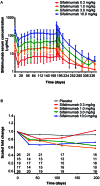
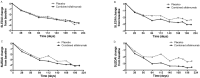
Results: Of 161 patients, 121 received sifalimumab (26 received 0.3 mg/kg; 25, 1.0 mg/kg; 27, 3.0 mg/kg; and 43, 10 mg/kg) and 40 received placebo. Patients were predominantly female (95.7%). At baseline, patients had moderate-to-severe disease activity (mean SLE Disease Activity Index score 11.0), and most (75.2%) had a high type I interferon (IFN) gene signature. In the sifalimumab group versus the placebo group, the incidence of ≥1 treatment-emergent AE was 92.6% versus 95.0%, ≥1 serious AE was 22.3% versus 27.5%, and ≥1 infection was 67.8% versus 62.5%; discontinuations due to AEs occurred in 9.1% versus 7.5%, and death occurred in 3.3% (n=4) versus 2.5% (n=1). Serum sifalimumab concentrations increased in a linear and dose-proportional manner. Inhibition of the type I IFN gene signature was sustained during treatment in patients with a high baseline signature. No statistically significant differences in clinical activity (SLEDAI and British Isles Lupus Assessment Group score) between sifalimumab and placebo were observed. However, when adjusted for excess burst steroids, SLEDAI change from baseline showed a positive trend over time. A trend toward normal complement C3 or C4 level at week 26 was seen in the sifalimumab groups compared with baseline.
Conclusion: The observed safety/tolerability and clinical activity profile of sifalimumab support its continued clinical development for SLE.
Trial registration: ClinicalTrials.gov NCT00482989.
Copyright © 2013 by the American College of Rheumatology.
Figures
Comment in
-
[Anti-interferon alpha therapy of systemic lupus erythematosus].Z Rheumatol. 2013 Oct;72(8):827-9. doi: 10.1007/s00393-013-1265-z. Z Rheumatol. 2013. PMID: 24043298 German. No abstract available.
References
-
- American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Guidelines for referral and management of systemic lupus erythematosus in adults. Arthritis Rheum. 1999;42:1785–96. - PubMed
-
- Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. - PubMed
-
- Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195–205. - PubMed
-
- Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. - PubMed
-
- To CH, Petri M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 2005;52:4003–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

