Imaging of endogenous exchangeable proton signals in the human brain using frequency labeled exchange transfer imaging
- PMID: 23400954
- PMCID: PMC3606647
- DOI: 10.1002/mrm.24655
Imaging of endogenous exchangeable proton signals in the human brain using frequency labeled exchange transfer imaging
Abstract
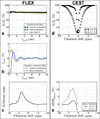
Purpose: To image endogenous exchangeable proton signals in the human brain using a recently reported method called frequency labeled exchange transfer (FLEX) MRI.
Methods: As opposed to labeling exchangeable protons using saturation (i.e., chemical exchange saturation transfer, or CEST), FLEX labels exchangeable protons with their chemical shift evolution. The use of short high-power frequency pulses allows more efficient labeling of rapidly exchanging protons, while time domain acquisition allows removal of contamination from semi-solid magnetization transfer effects.
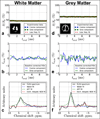
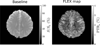
Results: FLEX-based exchangeable proton signals were detected in human brain over the 1-5 ppm frequency range from water. Conventional magnetization transfer contrast and the bulk water signal did not interfere in the FLEX spectrum. The information content of these signals differed from in vivo CEST data in that the average exchange rate of these signals was 350-400 s(-1) , much faster than the amide signal usually detected using direct saturation (∼30 s(-1) ). Similarly, fast exchanging protons could be detected in egg white in the same frequency range where amide and amine protons of mobile proteins and peptides are known to resonate.
Conclusions: FLEX MRI in the human brain preferentially detects more rapidly exchanging amide/amine protons compared to traditional CEST experiments, thereby changing the information content of the exchangeable proton spectrum. This has the potential to open up different types of endogenous applications as well as more easy detection of rapidly exchanging protons in diaCEST agents or fast exchanging units such as water molecules in paracest agents without interference of conventional magnetization transfer contrast.
Copyright © 2013 Wiley Periodicals, Inc.
Figures


References
-
- Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27:1129–1136. - PubMed
-
- Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–1090. - PubMed
-
- Ward K, Balaban R. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44:799–802. - PubMed
-
- Delli-Castelli D, Terreno E, Aime S. Ybiii-HPDO3A: A dual pH-and temperature-responsive CEST agent. Angew Chem Int Ed. 2011;50:1798–1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

