Microtubule-organizing center formation at telomeres induces meiotic telomere clustering
- PMID: 23401002
- PMCID: PMC3575533
- DOI: 10.1083/jcb.201207168
Microtubule-organizing center formation at telomeres induces meiotic telomere clustering
Abstract
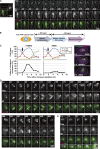
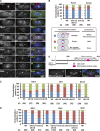
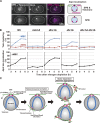
During meiosis, telomeres cluster and promote homologous chromosome pairing. Telomere clustering requires the interaction of telomeres with the nuclear membrane proteins SUN (Sad1/UNC-84) and KASH (Klarsicht/ANC-1/Syne homology). The mechanism by which telomeres gather remains elusive. In this paper, we show that telomere clustering in fission yeast depends on microtubules and the microtubule motors, cytoplasmic dynein, and kinesins. Furthermore, the γ-tubulin complex (γ-TuC) is recruited to SUN- and KASH-localized telomeres to form a novel microtubule-organizing center that we termed the "telocentrosome." Telocentrosome formation depends on the γ-TuC regulator Mto1 and on the KASH protein Kms1, and depletion of either Mto1 or Kms1 caused severe telomere clustering defects. In addition, the dynein light chain (DLC) contributes to telocentrosome formation, and simultaneous depletion of DLC and dynein also caused severe clustering defects. Thus, the telocentrosome is essential for telomere clustering. We propose that telomere-localized SUN and KASH induce telocentrosome formation and that subsequent microtubule motor-dependent aggregation of telocentrosomes via the telocentrosome-nucleated microtubules causes telomere clustering.
Figures


References
-
- Bähler J., Wu J.Q., Longtine M.S., Shah N.G., McKenzie A., III, Steever A.B., Wach A., Philippsen P., Pringle J.R. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 14:943–951 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

