The eye and the heart
- PMID: 23401492
- PMCID: PMC3640200
- DOI: 10.1093/eurheartj/eht023
The eye and the heart
Abstract
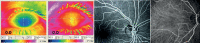
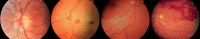
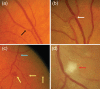
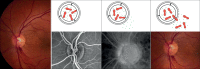
The vasculature of the eye and the heart share several common characteristics. The easily accessible vessels of the eye are therefore-to some extent-a window to the heart. There is interplay between cardiovascular functions and risk factors and the occurrence and progression of many eye diseases. In particular, arteriovenous nipping, narrowing of retinal arteries, and the dilatation of retinal veins are important signs of increased cardiovascular risk. The pressure in the dilated veins is often markedly increased due to a dysregulation of venous outflow from the eye. Besides such morphological criteria, functional alterations might be even more relevant and may play an important role in future diagnostics. Via neurovascular coupling, flickering light dilates capillaries and small arterioles, thus inducing endothelium-dependent, flow-mediated dilation of larger retinal vessels. Risk factors for arteriosclerosis, such as dyslipidaemia, diabetes, or systemic hypertension, are also risk factors for eye diseases such as retinal arterial or retinal vein occlusions, cataracts, age-related macular degeneration, and increases in intraocular pressure (IOP). Functional alterations of blood flow are particularly relevant to the eye. The primary vascular dysregulation syndrome (PVD), which often includes systemic hypotension, is associated with disturbed autoregulation of ocular blood flow (OBF). Fluctuation of IOP on a high level or blood pressure on a low level leads to instable OBF and oxygen supply and therefore to oxidative stress, which is particularly involved in the pathogenesis of glaucomatous neuropathy. Vascular dysregulation also leads to a barrier dysfunction and thereby to small retinal haemorrhages.
Keywords: Cardiovascular risk; Endothelial function; Glaucoma; Retinal vein occlusion; Retinal venous pressure; Retinal vessels; Systemic hypertension; Systemic hypotension; Vascular dysregulation.
Figures






References
-
- Mozaffarieh M, Flammer J. Ocular Blood Flow and Glaucomatous Optic Neuropathy. 1st ed. Berlin/Heidelberg: Springer; 2009.
-
- Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317–321. - PubMed
-
- Kotliar KE, Mucke B, Vilser W, Schilling R, Lanzl IM. Effect of aging on retinal artery blood column diameter measured along the vessel axis. Invest Ophthalmol Vis Sci. 2008;49:2094–2102. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

