Small molecule inhibitor of lipoteichoic acid synthesis is an antibiotic for Gram-positive bacteria
- PMID: 23401520
- PMCID: PMC3587227
- DOI: 10.1073/pnas.1217337110
Small molecule inhibitor of lipoteichoic acid synthesis is an antibiotic for Gram-positive bacteria
Abstract
The current epidemic of infections caused by antibiotic-resistant gram-positive bacteria requires the discovery of new drug targets and the development of new therapeutics. Lipoteichoic acid (LTA), a cell wall polymer of gram-positive bacteria, consists of 1,3-polyglycerol-phosphate linked to glycolipid. LTA synthase (LtaS) polymerizes polyglycerol-phosphate from phosphatidylglycerol, a reaction that is essential for the growth of gram-positive bacteria. We screened small molecule libraries for compounds inhibiting growth of Staphylococcus aureus but not of gram-negative bacteria. Compound 1771 [2-oxo-2-(5-phenyl-1,3,4-oxadiazol-2-ylamino)ethyl 2-naphtho[2,1-b]furan-1-ylacetate] blocked phosphatidylglycerol binding to LtaS and inhibited LTA synthesis in S. aureus and in Escherichia coli expressing ltaS. Compound 1771 inhibited the growth of antibiotic-resistant gram-positive bacteria and prolonged the survival of mice with lethal S. aureus challenge, validating LtaS as a target for the development of antibiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

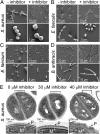
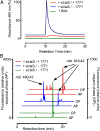

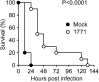
References
-
- Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. - PubMed
-
- Neu HC. The crisis in antibiotic resistance. Science. 1992;257(5073):1064–1073. - PubMed
-
- Payne DJ, Holmes DJ, Rosenberg M. Delivering novel targets and antibiotics from genomics. Curr Opin Investig Drugs. 2001;2(8):1028–1034. - PubMed
-
- Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6(5):427–430. - PubMed
-
- Walsh CT. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406(6797):775–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

