Reversal of age-related neural timing delays with training
- PMID: 23401541
- PMCID: PMC3600492
- DOI: 10.1073/pnas.1213555110
Reversal of age-related neural timing delays with training
Abstract
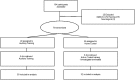
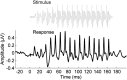
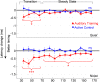
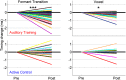
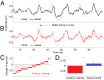
Neural slowing is commonly noted in older adults, with consequences for sensory, motor, and cognitive domains. One of the deleterious effects of neural slowing is impairment of temporal resolution; older adults, therefore, have reduced ability to process the rapid events that characterize speech, especially in noisy environments. Although hearing aids provide increased audibility, they cannot compensate for deficits in auditory temporal processing. Auditory training may provide a strategy to address these deficits. To that end, we evaluated the effects of auditory-based cognitive training on the temporal precision of subcortical processing of speech in noise. After training, older adults exhibited faster neural timing and experienced gains in memory, speed of processing, and speech-in-noise perception, whereas a matched control group showed no changes. Training was also associated with decreased variability of brainstem response peaks, suggesting a decrease in temporal jitter in response to a speech signal. These results demonstrate that auditory-based cognitive training can partially restore age-related deficits in temporal processing in the brain; this plasticity in turn promotes better cognitive and perceptual skills.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192(0):619–630. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

