Retinoic acid hypersensitivity promotes peripheral tolerance in recent thymic emigrants
- PMID: 23401586
- PMCID: PMC3594456
- DOI: 10.4049/jimmunol.1200852
Retinoic acid hypersensitivity promotes peripheral tolerance in recent thymic emigrants
Abstract
Whereas thymic education eliminates most self-reactive T cells, additional mechanisms to promote tolerance in the periphery are critical to prevent excessive immune responses against benign environmental Ags and some self-Ags. In this study we show that murine CD4(+) recent thymic emigrants (RTEs) are programmed to facilitate tolerance in the periphery. Both in vitro and in vivo, naive RTEs more readily upregulate Foxp3 than do mature naive cells after stimulation under tolerogenic conditions. In RTEs, a relatively high sensitivity to retinoic acid contributes to decreased IFN-γ production, permitting the expression of Foxp3. Conversely, mature naive CD4 cells have a lower sensitivity to retinoic acid, resulting in increased IFN-γ production and subsequent IFN-γ-mediated silencing of Foxp3 expression. Enhanced retinoic acid signaling and Foxp3 induction in RTEs upon Ag encounter in the periphery may serve as form of secondary education that complements thymic education and helps avoid inappropriate immune responses. This mechanism for tolerance may be particularly important in settings where RTEs comprise a large fraction of the peripheral T cell pool, such as in newborns or after umbilical cord blood transplant.
Figures
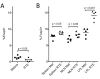
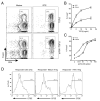
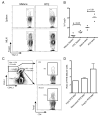

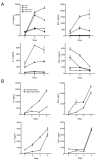


References
-
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. - PubMed
-
- Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. - PubMed
-
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. - PubMed
-
- Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. - PubMed
-
- Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25:303–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

