Further analysis of the lens of ephrin-A5-/- mice: development of postnatal defects
- PMID: 23401654
- PMCID: PMC3566898
Further analysis of the lens of ephrin-A5-/- mice: development of postnatal defects
Abstract
Purpose: The cells of the mammalian lens must be carefully organized and regulated to maintain clarity. Recent studies have identified the Eph receptor ligand ephrin-A5 as a major contributor to lens development, as mice lacking ephrin-A5 develop abnormal lenses, resulting in cataracts. As a follow-up to our previous study on the cataracts observed in ephrin-A5(-/-) animals, we have further examined the morphological and molecular changes in the ephrin-A5(-/-) lens.
Methods: Wild-type and ephrin-A5(-/-) eyes at various ages were fixed, sectioned, and examined using histological techniques. Protein expression and localization were determined using immunohistochemistry and western blot analysis.
Results: Lens abnormalities in the ephrin-A5(-/-) animals are observed at postnatal stages, with lens opacity occurring by postnatal day 21. Structural defects in the lens are first observed in the outer lens fiber cell region where cells in the ephrin-A5(-/-) lens are severely disorganized. Ephrin-A5 and the Eph receptor EphA2 are expressed during early ocular development and continue to be expressed into postnatal stages. The cataracts in the ephrin-A5(-/-) mutants occur regardless of the presence of the CP49 mutation.
Conclusions: In this follow-up study, we have uncovered additional details explicating the mechanisms underlying ephrin-A5 function in the lens. Furthermore, elucidation of the expression of ephrin-A5 and the Eph receptor EphA2 in the lens supports a fundamental role for this receptor-ligand complex in lens development. These observations, in concert with our previous study, strongly suggest that ephrin-A5 has a critical role in postnatal lens fiber organization to maintain lens transparency.
Figures

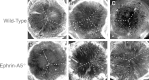
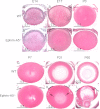







Similar articles
-
Diverse roles of Eph/ephrin signaling in the mouse lens.PLoS One. 2011;6(11):e28147. doi: 10.1371/journal.pone.0028147. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140528 Free PMC article.
-
EphA2 and ephrin-A5 are not a receptor-ligand pair in the ocular lens.Exp Eye Res. 2017 Sep;162:9-17. doi: 10.1016/j.exer.2017.06.016. Epub 2017 Jun 23. Exp Eye Res. 2017. PMID: 28648759 Free PMC article.
-
Mapping the Universe of Eph Receptor and Ephrin Ligand Transcripts in Epithelial and Fiber Cells of the Eye Lens.Cells. 2022 Oct 19;11(20):3291. doi: 10.3390/cells11203291. Cells. 2022. PMID: 36291158 Free PMC article.
-
Roles of Eph-Ephrin Signaling in the Eye Lens Cataractogenesis, Biomechanics, and Homeostasis.Front Cell Dev Biol. 2022 Feb 28;10:852236. doi: 10.3389/fcell.2022.852236. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35295853 Free PMC article. Review.
-
Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts.Handb Exp Pharmacol. 2009;(190):265-97. doi: 10.1007/978-3-540-79885-9_14. Handb Exp Pharmacol. 2009. PMID: 19096783 Review.
Cited by
-
Eph-ephrin Signaling Affects Eye Lens Fiber Cell Intracellular Voltage and Membrane Conductance.Front Physiol. 2021 Nov 25;12:772276. doi: 10.3389/fphys.2021.772276. eCollection 2021. Front Physiol. 2021. PMID: 34899394 Free PMC article.
-
EphA2 Affects Development of the Eye Lens Nucleus and the Gradient of Refractive Index.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):2. doi: 10.1167/iovs.63.1.2. Invest Ophthalmol Vis Sci. 2022. PMID: 34978559 Free PMC article.
-
Epha2 and Efna5 participate in lens cell pattern-formation.Differentiation. 2018 Jul-Aug;102:1-9. doi: 10.1016/j.diff.2018.05.002. Epub 2018 May 17. Differentiation. 2018. PMID: 29800803 Free PMC article.
-
Genotype, Age, Genetic Background, and Sex Influence Epha2-Related Cataract Development in Mice.Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):3. doi: 10.1167/iovs.62.12.3. Invest Ophthalmol Vis Sci. 2021. PMID: 34495288 Free PMC article.
-
EPHA2 MUTATIONS CONTRIBUTE TO CONGENITAL CATARACT THROUGH DIVERSE MECHANISMS.Mol Vis. 2016 Jan 14;22:18-30. eCollection 2016. Mol Vis. 2016. PMID: 26900323 Free PMC article.
References
-
- Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37:1396–410. - PubMed
-
- Shestopalov VI, Bassnett S. Three-dimensional organization of primary lens fiber cells. Invest Ophthalmol Vis Sci. 2000;41:859–63. - PubMed
-
- Bassnett S. Fiber cell denucleation in the primate lens. Invest Ophthalmol Vis Sci. 1997;38:1678–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
