Shotgun proteomic analysis of S-thiolation sites of guinea pig lens nuclear crystallins following oxidative stress in vivo
- PMID: 23401655
- PMCID: PMC3566901
Shotgun proteomic analysis of S-thiolation sites of guinea pig lens nuclear crystallins following oxidative stress in vivo
Abstract
Purpose: To compare levels of S-glutathiolation and S-cysteinylation occurring at more than 60 cysteine residues of 12 different guinea pig lens water-soluble nuclear crystallins following treatment of the animals with hyperbaric oxygen (HBO).
Methods: Guinea pigs (initially 18 months old) were treated 30X (3X per week for 10 weeks) with HBO (2.5 atm 100% O(2) for 2.5 h) as a model to study the formation of nuclear cataract. This treatment produces a moderate increase in lens nuclear light scatter (compared to denser scatter occurring after 80 HBO treatments), with five- to sixfold increases in levels of protein-bound glutathione (PSSG) and protein-bound cysteine (PSSC). Trypsin digests of lens nuclear water-soluble proteins were analyzed with two-dimensional liquid chromatography and mass spectrometry to identify specific cysteine residues binding either glutathione or cysteine. Lens nuclei of age-matched untreated animals were used as controls.
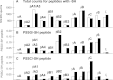
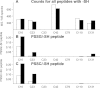
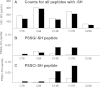
Results: All major crystallins, except αB, were modified to some extent by either S-glutathiolation or S-cysteinylation. Overall, 72% of the cysteine residues of guinea pig lens nuclear crystallins were shown to be capable of binding glutathione, cysteine, or both molecules. The crystallin with the highest level of modification was βA1/A3 (six of eight -SH groups), and that with the lowest (two of five -SH groups) was βA2. O(2)-induced increases in PSSG levels were 2.8, 2.4, and 4.1 times control for γA-, γB-, and γC-crystallins, respectively. Comparable increases in PSSC levels for the three γ-crystallins were 2.3, 2.7, and 2.4 times control, respectively. βB2-crystallin showed the highest amount of O(2)-induced PSSG formation of any of the crystallins, as well as a substantial level of control PSSG, and nearly all of this was due to a single residue, C67, a site also present in human βB2-crystallin. Overall, 32 of the 44 modified cysteine residues were homologous with the human.
Conclusions: This large-scale study successfully identified lens crystallin cysteine residues that bound glutathione and/or cysteine under normal or oxidative stress conditions. The high percentage of protein -SH groups that are modified by S-thiolation in the guinea pig lens nucleus demonstrates the substantial protein sulfhydryl redox buffer capability present in the center of the lens. The results suggest that PSSG and PSSC formation may act to delay O(2)-induced insolubilization of γA-, γB-, and γC-crystallins, and β-crystallins, but with a greater effect on the γ-crystallins at an early stage of oxidative stress. The study has shown that technological approaches are now available to investigate in considerable detail the role of specific lens -SH groups in nuclear cataractogenesis.
Figures


Similar articles
-
Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis.Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4641-51. doi: 10.1167/iovs.05-0843. Invest Ophthalmol Vis Sci. 2005. PMID: 16303961 Free PMC article.
-
The effects of hyperbaric oxygen on the crystallins of cultured rabbit lenses: a possible catalytic role for copper.Exp Eye Res. 2000 Oct;71(4):371-83. doi: 10.1006/exer.2000.0887. Exp Eye Res. 2000. PMID: 10995558
-
Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen.Exp Eye Res. 1995 Mar;60(3):219-35. doi: 10.1016/s0014-4835(05)80105-8. Exp Eye Res. 1995. PMID: 7789403
-
Redox regulation in the lens.Prog Retin Eye Res. 2003 Sep;22(5):657-82. doi: 10.1016/s1350-9462(03)00050-8. Prog Retin Eye Res. 2003. PMID: 12892645 Review.
-
Thiol regulation in the lens.J Ocul Pharmacol Ther. 2000 Apr;16(2):137-48. doi: 10.1089/jop.2000.16.137. J Ocul Pharmacol Ther. 2000. PMID: 10803424 Review.
Cited by
-
Role of αA-crystallin-derived αA66-80 peptide in guinea pig lens crystallin aggregation and insolubilization.Exp Eye Res. 2015 Mar;132:151-60. doi: 10.1016/j.exer.2015.01.024. Epub 2015 Jan 29. Exp Eye Res. 2015. PMID: 25639202 Free PMC article.
-
Antioxidant System and Endoplasmic Reticulum Stress in Cataracts.Cell Mol Neurobiol. 2023 Nov;43(8):4041-4058. doi: 10.1007/s10571-023-01427-4. Epub 2023 Oct 24. Cell Mol Neurobiol. 2023. PMID: 37874455 Free PMC article. Review.
-
Oxidation-Induced Mixed Disulfide and Cataract Formation: A Review.Antioxidants (Basel). 2025 Apr 1;14(4):425. doi: 10.3390/antiox14040425. Antioxidants (Basel). 2025. PMID: 40298781 Free PMC article. Review.
-
ROLE OF THIOLS IN OXIDATIVE STRESS.Curr Opin Toxicol. 2018 Feb;7:133-139. doi: 10.1016/j.cotox.2018.03.005. Epub 2018 Mar 21. Curr Opin Toxicol. 2018. PMID: 30338308 Free PMC article.
-
Trioxidized cysteine in the aging proteome mimics the structural dynamics and interactome of phosphorylated serine.Aging Cell. 2024 Mar;23(3):e14062. doi: 10.1111/acel.14062. Epub 2023 Dec 18. Aging Cell. 2024. PMID: 38111315 Free PMC article.
References
-
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–35. - PubMed
-
- Kuck JF, Yu NT, Askren CC. Total sulfhydryl by raman spectroscopy in the intact lens of several species: variations in the nucleus and along the optical axis during aging. Exp Eye Res. 1982;34:23–37. - PubMed
-
- Giblin FJ, Reddy VN. High molecular weight protein aggregates in X-ray-induced cataract: evidence for the involvement of disulfide bonds In: von Hahn HP, Basel, editors. Interdisciplinary Topics in Gerontology. Vol 12. Switzerland: S. Karger, Basel; 1978. p. 94–104.
-
- Srikanthan D, Bateman OA, Purkiss AG, Slingsby C. Sulfur in human crystallins. Exp Eye Res. 2004;79:823–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
