Cyclin-dependent kinase 5 promotes the stability of corneal epithelial cell junctions
- PMID: 23401660
- PMCID: PMC3566902
Cyclin-dependent kinase 5 promotes the stability of corneal epithelial cell junctions
Abstract
Purpose: Although cyclin-dependent kinase 5 (Cdk5) inhibits the formation of junctions containing N-cadherin, the effect of Cdk5 on junctions containing E-cadherin is less clear. The present study investigates the functional significance of Cdk5 in forming and maintaining cell-cell stability in corneal epithelial cells.
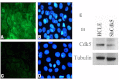
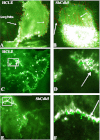
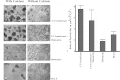
Methods: A Cdk5-deficient human corneal limbal epithelial cell line was generated by lentiviral transduction of small hairpin RNA specific for Cdk5 (shCdk5-HCLE cells). A blasticidin-inducible vector for expression of Cdk5-specific short hairpin RNA (ShCdk5) was generated by recombination and packaged into non-replicative lentiviral particles for transduction of human corneal limbal epithelial (HCLE) cells. Blasticidin-resistant cells were isolated for analysis. Cell aggregations were performed using HCLE, Cdk5 inhibitor olomoucine, ShCdk5, and MDA-MB 231 cells in the presence and absence of calcium, and particle size was measured using image analysis software. Relative protein concentrations were measured with immunoblotting and quantitative densitometry. Total internal reflection fluorescence (TIRF) microscopy was performed on cells transfected with green fluorescent protein (GFP)-E-cadherin or GFP-p120, and internalization of boundary-localized proteins was analyzed with particle tracking software. The stability of surface-exposed proteins was determined by measuring the recovery of biotin-labeled proteins with affinity chromatography. Rho and Rac activity was measured with affinity chromatography and immunoblotting.
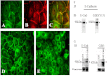
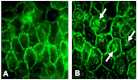
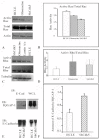
Results: Examining the effect of Cdk5 on E-cadherin containing epithelial cell-cell adhesions using a corneal epithelial cell line (HCLE), we found that Cdk5 and Cdk5 (pY15) coimmunoprecipitate with E-cadherin and Cdk5 (pY15) colocalizes with E-cadherin at cell-cell junctions. Inhibiting Cdk5 activity in HCLE or suppressing Cdk5 expression in a stable HCLE-derived cell line (ShHCLE) decreased calcium-dependent cell adhesion, promoted the cytoplasmic localization of E-cadherin, and accelerated the loss of surface-biotinylated E-cadherin. TIRF microscopy of GFP-E-cadherin in transfected HCLE cells showed an actively internalized sub-population of E-cadherin, which was not bound to p120 as it was trafficked away from the cell-cell boundary. This population increased in the absence of Cdk5 activity, suggesting that Cdk5 inhibition promotes dissociation of p120/E-cadherin junctional complexes. These effects of Cdk5 inhibition or suppression were accompanied by decreased Rac activity, increased Rho activity, and enhanced binding of E-cadherin to the Rac effector Ras GTPase-activating-like protein (IQGAP1). Cdk5 inhibition also reduced adhesion in a cadherin-deficient cell line (MDA-MB-231) expressing exogenous E-cadherin, although Cdk5 inhibition promoted adhesion when these cells were transfected with N-cadherin, as previous studies of Cdk5 and N-cadherin predicted. Moreover, Cdk5 inhibition induced N-cadherin expression and formation of N-cadherin/p120 complexes in HCLE cells.
Conclusions: These results indicate that loss of Cdk5 activity destabilizes junctional complexes containing E-cadherin, leading to internalization of E-cadherin and upregulation of N-cadherin. Thus, Cdk5 activity promotes stability of E-cadherin-based cell-cell junctions and inhibits the E-cadherin-to-N-cadherin switch typical of epithelial-mesenchymal transitions.
Figures

References
-
- Meyerson G, Pfenninger KH, Påhlman S. A complex consisting of pp60c-src/pp60c-srcN and a 38 kDa protein is highly enriched in growth cones from differentiated SH-SY5Y neuroblastoma cells. J Cell Sci. 1992;103:233–43. - PubMed
-
- Gao CY, Stepp MA, Fariss R, Zelenka P. Cdk5 regulates activation and localization of Src during corneal epithelial wound closure. J Cell Sci. 2004;117:4089–98. - PubMed
-
- Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28:1023–34. Review. - PubMed
-
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–23. - PubMed
-
- Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
