An endothelialized urothelial cell-seeded tubular graft for urethral replacement
- PMID: 23401738
- PMCID: PMC3559612
- DOI: 10.5489/cuaj.12217
An endothelialized urothelial cell-seeded tubular graft for urethral replacement
Abstract
Introduction: Many efforts are used to improve surgical techniques and graft materials for urethral reconstruction. We developed an endothelialized tubular structure for urethral reconstruction.
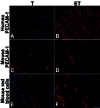
Methods: Two tubular models were created in vitro. Human fibroblasts were cultured for 4 weeks to form fibroblast sheets. Then, endothelial cells (ECs) were seeded on the fibroblast sheets and wrapped around a tubular support to form a cylinder for the endothelialized tubular urethral model (ET). No ECs were added in the standard tubular model (T). After 21 days of maturation, urothelial cells were seeded into the lumen of both models. Constructs were placed under perfusion in a bioreactor for 1 week. At several times, histology and immunohistochemistry were performed on grafted nude mice to evaluate the impact of ECs on vascularization.
Results: Both models produced an extracellular matrix, without exogenous material, and developed a pseudostratified urothelium. Seven days after the graft, mouse red blood cells were present only in the outer layers in T model, but in the full thickness of ET model. After 14 days, erythrocytes were present in both models, but in a greater proportion in ET model. At day 28, both models were well-vascularized, with capillary-like structures in the whole thickness of the tubes.
Conclusion: Incorporating endothelial cells was associated with an earlier vascularization of the grafts, which could decrease the necrosis of the transplanted tissue. As those models can be elaborated with the patient's cells, this tubular urethral graft would be unique in its autologous property.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
