CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease
- PMID: 23401787
- PMCID: PMC3564380
- DOI: 10.1155/2013/614619
CD47: A Cell Surface Glycoprotein Which Regulates Multiple Functions of Hematopoietic Cells in Health and Disease
Abstract
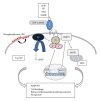
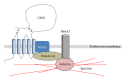
Interactions between cells and their surroundings are important for proper function and homeostasis in a multicellular organism. These interactions can either be established between the cells and molecules in their extracellular milieu, but also involve interactions between cells. In all these situations, proteins in the plasma membranes are critically involved to relay information obtained from the exterior of the cell. The cell surface glycoprotein CD47 (integrin-associated protein (IAP)) was first identified as an important regulator of integrin function, but later also was shown to function in ways that do not necessarily involve integrins. Ligation of CD47 can induce intracellular signaling resulting in cell activation or cell death depending on the exact context. By binding to another cell surface glycoprotein, signal regulatory protein alpha (SIRPα), CD47 can regulate the function of cells in the monocyte/macrophage lineage. In this spotlight paper, several functions of CD47 will be reviewed, although some functions may be more briefly mentioned. Focus will be on the ways CD47 regulates hematopoietic cells and functions such as CD47 signaling, induction of apoptosis, and regulation of phagocytosis or cell-cell fusion.
Figures


References
-
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274(5288):795–798. - PubMed
-
- Lindberg FP, Lublin DM, Telen MJ, et al. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. Journal of Biological Chemistry. 1994;269(3):1567–1570. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

