Hemostatic challenges in patients with chronic immune thrombocytopenia treated with eltrombopag
- PMID: 23402314
- PMCID: PMC3913069
- DOI: 10.3109/09537104.2013.764980
Hemostatic challenges in patients with chronic immune thrombocytopenia treated with eltrombopag
Abstract
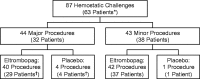
Chronic immune thrombocytopenia (ITP) is an autoimmune disease that results in chronically low platelet counts. Treatment guidelines recommend a platelet count of at least 50,000/µl before minor surgery and at least 80,000/µl before major surgery. This retrospective analysis explored invasive non-dental procedures associated with the risk of bleeding (hemostatic challenges) among patients with chronic ITP in five phase 2/phase 3 studies of the thrombopoietin-receptor agonist, eltrombopag. Data collection for patients who underwent hemostatic challenges included demographics, study medication, timing of the procedure, platelet counts at last assessment before and first assessment after the procedure, supplemental ITP treatment, and bleeding events. Among 494 patients who participated in the studies, 87 hemostatic challenges were recorded. Median platelet counts before 44 major procedures in 32 patients were 100,000/µl and 18,500/µl among patients who received eltrombopag and placebo, respectively; before 43 minor procedures in 38 patients, median platelet counts were 82,000/µl and 20,000/µl among patients who received eltrombopag and placebo, respectively. A minority of patients required supplemental ITP treatment. Only 2 of 87 hemostatic challenges were associated with bleeding events; both patients received eltrombopag and pre-procedural platelet counts were 83,000/µl and 2000/µl. Although the number of patients who did not undergo procedures due to thrombocytopenia was not captured, these data suggest a majority of patients with chronic ITP who receive eltrombopag and experience increases in platelet counts meet current pre-procedural platelet count recommendations. The potential role of eltrombopag in supporting preparation of chronic ITP patients for surgical procedures still needs to be clinically established.
Figures
Similar articles
-
Dental procedures in 24 patients with chronic immune thrombocytopenia in prospective clinical studies of eltrombopag.Platelets. 2015;26(1):93-6. doi: 10.3109/09537104.2013.870333. Epub 2014 Jan 16. Platelets. 2015. PMID: 24433306
-
Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial.Lancet. 2009 Feb 21;373(9664):641-8. doi: 10.1016/S0140-6736(09)60402-5. Lancet. 2009. PMID: 19231632 Clinical Trial.
-
Eltrombopag: A Review in Paediatric Chronic Immune Thrombocytopenia.Drugs. 2016 May;76(8):869-78. doi: 10.1007/s40265-016-0581-4. Drugs. 2016. PMID: 27151255 Review.
-
Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial.Lancet. 2015 Oct 24;386(10004):1649-58. doi: 10.1016/S0140-6736(15)61107-2. Epub 2015 Jul 28. Lancet. 2015. PMID: 26231455 Clinical Trial.
-
Eltrombopag: a review of its use in treatment-refractory chronic primary immune thrombocytopenia.Drugs. 2011 Jul 9;71(10):1333-53. doi: 10.2165/11207390-000000000-00000. Drugs. 2011. PMID: 21770480 Review.
Cited by
-
Dentoalveolar Procedures in Immune Thrombocytopenia; Systematic Review and an Institutional Guideline.TH Open. 2021 Sep 9;5(4):e489-e502. doi: 10.1055/a-1641-7770. eCollection 2021 Oct. TH Open. 2021. PMID: 34805736 Free PMC article. Review.
-
The role of eltrombopag in the management of hepatitis C virus-related thrombocytopenia.Hepat Med. 2013 Mar 15;5:17-30. doi: 10.2147/HMER.S27100. eCollection 2013. Hepat Med. 2013. PMID: 24696622 Free PMC article. Review.
References
-
- Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205–213. - PubMed
-
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A. Solberg Jr. L, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–4207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
