An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity
- PMID: 23402317
- PMCID: PMC3602176
- DOI: 10.1186/1743-422X-10-52
An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity
Abstract
Background: Lassa hemorrhagic fever (LHF) is a rodent-borne viral disease that can be fatal for human beings. In this study, an attenuated Lassa vaccine candidate, ML29, was tested in SIV-infected rhesus macaques for its ability to elicit immune responses without instigating signs pathognomonic for arenavirus disease. ML29 is a reassortant between Lassa and Mopeia viruses that causes a transient infection in non-human primates and confers sterilizing protection from lethal Lassa viral challenge. However, since the LHF endemic area of West Africa also has high HIV seroprevalence, it is important to determine whether vaccination could be safe in the context of HIV infection.
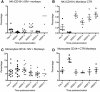
Results: SIV-infected and uninfected rhesus macaques were vaccinated with the ML29 virus and monitored for specific humoral and cellular immune responses, as well as for classical and non-classical signs of arenavirus disease. Classical disease signs included viremia, rash, respiratory distress, malaise, high liver enzyme levels, and virus invasion of the central nervous system. Non-classical signs, derived from profiling the blood transcriptome of virulent and non-virulent arenavirus infections, included increased expression of interferon-stimulated genes (ISG) and decreased expression of COX2, IL-1β, coagulation intermediates and nuclear receptors needed for stress signaling. All vaccinated monkeys showed ML29-specific antibody responses and ML29-specific cell-mediated immunity.
Conclusion: SIV-infected and uninfected rhesus macaques responded similarly to ML29 vaccination, and none developed chronic arenavirus infection. Importantly, none of the macaques developed signs, classical or non-classical, of arenavirus disease.
Figures





Similar articles
-
Genetic variation in vitro and in vivo of an attenuated Lassa vaccine candidate.J Virol. 2014 Mar;88(6):3058-66. doi: 10.1128/JVI.03035-13. Epub 2013 Dec 11. J Virol. 2014. PMID: 24335292 Free PMC article.
-
A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses.J Virol. 2005 Nov;79(22):13934-42. doi: 10.1128/JVI.79.22.13934-13942.2005. J Virol. 2005. PMID: 16254329 Free PMC article.
-
Lassa Virus Vaccine Candidate ML29 Generates Truncated Viral RNAs Which Contribute to Interfering Activity and Attenuation.Viruses. 2021 Jan 30;13(2):214. doi: 10.3390/v13020214. Viruses. 2021. PMID: 33573250 Free PMC article.
-
Towards a human Lassa fever vaccine.Rev Med Virol. 2001 Sep-Oct;11(5):331-41. doi: 10.1002/rmv.329. Rev Med Virol. 2001. PMID: 11590670 Review.
-
Vaccine platforms to control Lassa fever.Expert Rev Vaccines. 2016 Sep;15(9):1135-50. doi: 10.1080/14760584.2016.1184575. Epub 2016 May 24. Expert Rev Vaccines. 2016. PMID: 27136941 Review.
Cited by
-
Depletion of CD4 and CD8 T Cells Reduces Acute Disease and Is Not Associated with Hearing Loss in ML29-Infected STAT1-/- Mice.Biomedicines. 2022 Sep 29;10(10):2433. doi: 10.3390/biomedicines10102433. Biomedicines. 2022. PMID: 36289695 Free PMC article.
-
A Lassa Fever Live-Attenuated Vaccine Based on Codon Deoptimization of the Viral Glycoprotein Gene.mBio. 2020 Feb 25;11(1):e00039-20. doi: 10.1128/mBio.00039-20. mBio. 2020. PMID: 32098811 Free PMC article.
-
Transcriptome analysis of human peripheral blood mononuclear cells exposed to Lassa virus and to the attenuated Mopeia/Lassa reassortant 29 (ML29), a vaccine candidate.PLoS Negl Trop Dis. 2013 Sep 12;7(9):e2406. doi: 10.1371/journal.pntd.0002406. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24069471 Free PMC article.
-
Adjuvants Differentially Modulate the Immunogenicity of Lassa Virus Glycoprotein Subunits in Mice.Front Trop Dis. 2022;3:847598. doi: 10.3389/fitd.2022.847598. Epub 2022 Mar 10. Front Trop Dis. 2022. PMID: 37034031 Free PMC article.
-
Lassa Virus Countermeasures.Curr Top Microbiol Immunol. 2023;440:111-145. doi: 10.1007/82_2022_261. Curr Top Microbiol Immunol. 2023. PMID: 36253593
References
-
- Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. - DOI - PubMed
-
- Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, Tordo N, Deubel V, Contamin H. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J Virol. 2009;83:5890–5903. doi: 10.1128/JVI.01948-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

