The contribution of N₂O₃ to the cytotoxicity of the nitric oxide donor DETA/NO: an emerging role for S-nitrosylation
- PMID: 23402389
- PMCID: PMC3610299
- DOI: 10.1042/BSR20120120
The contribution of N₂O₃ to the cytotoxicity of the nitric oxide donor DETA/NO: an emerging role for S-nitrosylation
Abstract
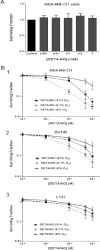
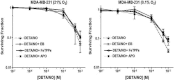
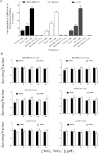
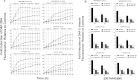
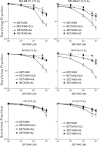
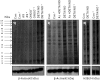
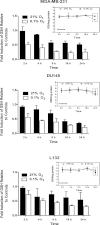
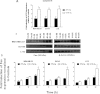
The relationship between the biological activity of NO and its chemistry is complex. The objectives of this study were to investigate the influence of oxygen tension on the cytotoxicity of the NO• donor DETA/NO and to determine the effects of oxygen tension on the key RNS (reactive nitrogen species) responsible for any subsequent toxicity. The findings presented in this study indicate that the DETA/NO-mediated cytotoxic effects were enhanced under hypoxic conditions. Further investigations revealed that neither ONOO⁻ (peroxynitrite) nor nitroxyl was generated. Fluorimetric analysis in the presence of scavengers suggest for the first time that another RNS, dinitrogen trioxide may be responsible for the cytotoxicity with DETA/NO. Results showed destabilization of HIF (hypoxia inducible factor)-1α and depletion of GSH levels following the treatment with DETA/NO under hypoxia, which renders cells more susceptible to DETA/NO cytotoxicity, and could account for another mechanism of DETA/NO cytotoxicity under hypoxia. In addition, there was significant accumulation of nuclear p53, which showed that p53 itself might be a target for S-nitrosylation following the treatment with DETA/NO. Both the intrinsic apoptotic pathway and the Fas extrinsic apoptotic pathway were also activated. Finally, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) is another important S-nitrosylated protein that may possibly play a key role in DETA/NO-mediated apoptosis and cytotoxicity. Therefore this study elucidates further mechanisms of DETA/NO mediated cytotoxicity with respect to S-nitrosylation that is emerging as a key player in the signalling and detection of DETA/NO-modified proteins in the tumour microenvironment.
Figures

Similar articles
-
Accumulation of HIF-1alpha under the influence of nitric oxide.Blood. 2001 Feb 15;97(4):1009-15. doi: 10.1182/blood.v97.4.1009. Blood. 2001. PMID: 11159530
-
Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways.J Biol Chem. 2004 Feb 6;279(6):4425-32. doi: 10.1074/jbc.M310582200. Epub 2003 Nov 3. J Biol Chem. 2004. PMID: 14597620
-
NO restores HIF-1alpha hydroxylation during hypoxia: role of reactive oxygen species.Free Radic Biol Med. 2005 Oct 1;39(7):925-36. doi: 10.1016/j.freeradbiomed.2005.05.009. Free Radic Biol Med. 2005. PMID: 16140212
-
HIF-1alpha and p53 as targets of NO in affecting cell proliferation, death and adaptation.Curr Mol Med. 2004 Nov;4(7):741-51. doi: 10.2174/1566524043359926. Curr Mol Med. 2004. PMID: 15579021 Review.
-
Mechanisms of hypoxic signal transduction regulated by reactive nitrogen species.Scand J Immunol. 2007 May;65(5):399-406. doi: 10.1111/j.1365-3083.2007.01919.x. Scand J Immunol. 2007. PMID: 17444949 Review.
Cited by
-
Regeneration in the nervous system with erythropoietin.Front Biosci (Landmark Ed). 2016 Jan;21(3):561-596. doi: 10.2741/4408. Front Biosci (Landmark Ed). 2016. PMID: 26549969 Free PMC article.
-
The effect of the release of exogenous nitric oxide on the responses of the pregnant human myometrium to oxytocin.Dev Period Med. 2018;22(4):301-307. doi: 10.34763/devperiodmed.20182204.301307. Dev Period Med. 2018. PMID: 30636226 Free PMC article.
-
Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection.Antioxidants (Basel). 2024 Apr 23;13(5):504. doi: 10.3390/antiox13050504. Antioxidants (Basel). 2024. PMID: 38790609 Free PMC article. Review.
-
Systemic RALA/iNOS Nanoparticles: A Potent Gene Therapy for Metastatic Breast Cancer Coupled as a Biomarker of Treatment.Mol Ther Nucleic Acids. 2017 Mar 17;6:249-258. doi: 10.1016/j.omtn.2016.12.010. Epub 2016 Dec 31. Mol Ther Nucleic Acids. 2017. PMID: 28325291 Free PMC article.
-
Albumin is an interface between blood plasma and cell membrane, and not just a sponge.Clin Kidney J. 2021 Oct 5;15(4):624-634. doi: 10.1093/ckj/sfab194. eCollection 2022 Apr. Clin Kidney J. 2021. PMID: 35371452 Free PMC article.
References
-
- Lancaster J. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. - PubMed
-
- Ying L., Hofseth L. J. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–1415. - PubMed
-
- Hirst D., Robson T. Targeting nitric oxide for cancer therapy. J. Pharm. Pharmacol. 2007;59:3–13. - PubMed
-
- Shami P. J., Saavedra J. E., Bonifant C. L., Chu J., Udupi V., Malaviya S., Carr B. I., Kar S., Wang M., Jia L. Antitumor activity of JS-K [O2-(2, 4-dinitrophenyl) 1-[(4-ethoxycarbonyl) piperazin-1-yl] diazen-1-ium-1, 2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J. Med. Chem. 2006;49:4356–4366. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

