High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro
- PMID: 23402570
- PMCID: PMC3579702
- DOI: 10.1186/1556-276X-8-72
High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro
Abstract
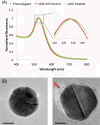
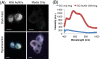
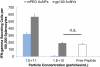
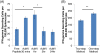
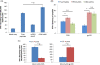
Nanocarriers have been explored to improve the delivery of tumor antigens to dendritic cells (DCs). Gold nanoparticles are attractive nanocarriers because they are inert, non-toxic, and can be readily endocytosed by DCs. Here, we designed novel gold-based nanovaccines (AuNVs) using a simple self-assembling bottom-up conjugation method to generate high-peptide density delivery and effective immune responses with limited toxicity. AuNVs were synthesized using a self-assembling conjugation method and optimized using DC-to-splenocyte interferon-γ enzyme-linked immunosorbent spot assays. The AuNV design has shown successful peptide conjugation with approximately 90% yield while remaining smaller than 80 nm in diameter. DCs uptake AuNVs with minimal toxicity and are able to process the vaccine peptides on the particles to stimulate cytotoxic T lymphocytes (CTLs). These high-peptide density AuNVs can stimulate CTLs better than free peptides and have great potential as carriers for various vaccine types.
Figures

Similar articles
-
Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation.ACS Appl Mater Interfaces. 2013 May;5(9):3900-7. doi: 10.1021/am400590m. Epub 2013 Apr 22. ACS Appl Mater Interfaces. 2013. PMID: 23566248
-
PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses.Int J Nanomedicine. 2012;7:1475-87. doi: 10.2147/IJN.S29506. Epub 2012 Mar 15. Int J Nanomedicine. 2012. PMID: 22619507 Free PMC article.
-
Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy.Mol Pharm. 2011 Feb 7;8(1):104-16. doi: 10.1021/mp100178k. Epub 2010 Dec 20. Mol Pharm. 2011. PMID: 21121669
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Dendritic cells reconstituted with a human heparanase gene induce potent cytotoxic T-cell responses against gastric tumor cells in vitro.Tumour Biol. 2007;28(4):238-46. doi: 10.1159/000107584. Epub 2007 Aug 23. Tumour Biol. 2007. PMID: 17717429
Cited by
-
Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines.Cancers (Basel). 2020 Apr 23;12(4):1049. doi: 10.3390/cancers12041049. Cancers (Basel). 2020. PMID: 32340356 Free PMC article. Review.
-
Strategies on Nanodiagnostics and Nanotherapies of the Three Common Cancers.Nanomaterials (Basel). 2018 Mar 28;8(4):202. doi: 10.3390/nano8040202. Nanomaterials (Basel). 2018. PMID: 29597315 Free PMC article. Review.
-
Leveraging Tunable Nanoparticle Surface Functionalization to Alter Cellular Migration.ACS Nanosci Au. 2024 Feb 14;4(3):205-215. doi: 10.1021/acsnanoscienceau.3c00055. eCollection 2024 Jun 19. ACS Nanosci Au. 2024. PMID: 38912285 Free PMC article.
-
Unlocking the Potential of Gold as Nanomedicine in Cancer Immunotherapy.J Nanotheranostics. 2024 Jun;5(2):29-59. doi: 10.3390/jnt5020003. Epub 2024 Apr 30. J Nanotheranostics. 2024. PMID: 40808797 Free PMC article.
-
Gold nanoparticle mediated cancer immunotherapy.Nanomedicine. 2014 Apr;10(3):503-14. doi: 10.1016/j.nano.2013.09.011. Epub 2013 Oct 5. Nanomedicine. 2014. PMID: 24103304 Free PMC article. Review.
References
-
- Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

