Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer
- PMID: 23402593
- PMCID: PMC3609912
- DOI: 10.1021/nn3043463
Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer
Abstract
The tumor stroma in human cancers significantly limits the delivery of therapeutic agents into cancer cells. To develop an effective therapeutic approach overcoming the physical barrier of the stroma, we engineered urokinase plasminogen activator receptor (uPAR)-targeted magnetic iron oxide nanoparticles (IONPs) carrying chemotherapy drug gemcitabine (Gem) for targeted delivery into uPAR-expressing tumor and stromal cells. The uPAR-targeted nanoparticle construct, ATF-IONP-Gem, was prepared by conjugating IONPs with the amino-terminal fragment (ATF) peptide of the receptor-binding domain of uPA, a natural ligand of uPAR, and Gem via a lysosomally cleavable tetrapeptide linker. These theranostic nanoparticles enable intracellular release of Gem following receptor-mediated endocytosis of ATF-IONP-Gem into tumor cells and also provide contrast enhancement in magnetic resonance imaging (MRI) of tumors. Our results demonstrated the pH- and lysosomal enzyme-dependent release of gemcitabine, preventing the drug from enzymatic degradation. Systemic administrations of ATF-IONP-Gem significantly inhibited the growth of orthotopic human pancreatic cancer xenografts in nude mice. With MRI contrast enhancement by IONPs, we detected the presence of IONPs in the residual tumors following the treatment, suggesting the possibility of monitoring drug delivery and assessing drug-resistant tumors by MRI. The theranostic ATF-IONP-Gem nanoparticle has great potential for the development of targeted therapeutic and imaging approaches that are capable of overcoming the tumor stromal barrier, thus enhancing the therapeutic effect of nanoparticle drugs on pancreatic cancers.
Figures
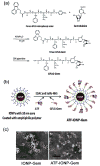

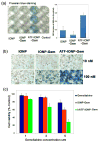
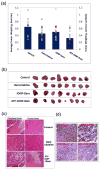



Similar articles
-
Functionalized milk-protein-coated magnetic nanoparticles for MRI-monitored targeted therapy of pancreatic cancer.Int J Nanomedicine. 2016 Jul 7;11:3087-99. doi: 10.2147/IJN.S92722. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27462153 Free PMC article.
-
A Nanoparticle Carrier for Co-Delivery of Gemcitabine and Small Interfering RNA in Pancreatic Cancer Therapy.J Biomed Nanotechnol. 2016 Aug;12(8):1654-66. doi: 10.1166/jbn.2016.2269. J Biomed Nanotechnol. 2016. PMID: 29342344
-
IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer.ACS Nano. 2015 Aug 25;9(8):7976-91. doi: 10.1021/acsnano.5b01288. Epub 2015 Aug 10. ACS Nano. 2015. PMID: 26242412 Free PMC article.
-
Nanotechnology for delivery of gemcitabine to treat pancreatic cancer.Biomed Pharmacother. 2017 Apr;88:635-643. doi: 10.1016/j.biopha.2017.01.071. Epub 2017 Jan 28. Biomed Pharmacother. 2017. PMID: 28142120 Review.
-
Acquired and intrinsic gemcitabine resistance in pancreatic cancer therapy: Environmental factors, molecular profile and drug/nanotherapeutic approaches.Environ Res. 2024 Jan 1;240(Pt 2):117443. doi: 10.1016/j.envres.2023.117443. Epub 2023 Oct 18. Environ Res. 2024. PMID: 37863168 Review.
Cited by
-
Selecting Tumor-Specific Molecular Targets in Pancreatic Adenocarcinoma: Paving the Way for Image-Guided Pancreatic Surgery.Mol Imaging Biol. 2016 Dec;18(6):807-819. doi: 10.1007/s11307-016-0959-4. Mol Imaging Biol. 2016. PMID: 27130234 Free PMC article.
-
Nano-engineered delivery systems for cancer imaging and therapy: Recent advances, future direction and patent evaluation.Drug Discov Today. 2019 Feb;24(2):462-491. doi: 10.1016/j.drudis.2018.08.009. Epub 2018 Aug 16. Drug Discov Today. 2019. PMID: 30121330 Free PMC article. Review.
-
FGF2 engineered SPIONs attenuate tumor stroma and potentiate the effect of chemotherapy in 3D heterospheroidal model of pancreatic tumor.Nanotheranostics. 2020 Jan 1;4(1):26-39. doi: 10.7150/ntno.38092. eCollection 2020. Nanotheranostics. 2020. PMID: 31911892 Free PMC article.
-
Pancreatic Cancer: Recent Advances in Nanoformulation-Based Therapies.Crit Rev Ther Drug Carrier Syst. 2019;36(1):59-91. doi: 10.1615/CritRevTherDrugCarrierSyst.2018025459. Crit Rev Ther Drug Carrier Syst. 2019. PMID: 30806206 Free PMC article.
-
Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles.Theranostics. 2019 Jan 24;9(3):778-795. doi: 10.7150/thno.29964. eCollection 2019. Theranostics. 2019. PMID: 30809308 Free PMC article.
References
-
- Willett CG, Czito BG, Bendell JC, Ryan DP. Locally Advanced Pancreatic Cancer. J Clin Oncol. 2005;23:4538–4544. - PubMed
-
- Warshaw AL, Fernandez-del Castillo C. Pancreatic Carcinoma. N Engl J Med. 1992;326:455–465. - PubMed
-
- Mahadevan D, Von Hoff DD. Tumor-Stroma Interactions in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in Cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

